- European Medicines Agency Initiates Finasteride-Safety Probe Focused Solely on Suicidality—which Could Lead to Label Changes, Market Suspension or Market Withdrawal in 30 NationsAll 200 pharma firms selling 5-ARIs in the EU must address ‘causal relationship’ between drugs’ use and patientsconsidering, attempting or committing suicide Oct. 11, 2024 Dear Friends: Depending on how deadly regulators across the pond find finasteride to be, the drug could be in for a continental shelving come February. Countdown to D-Day Last week,… Read more: European Medicines Agency Initiates Finasteride-Safety Probe Focused Solely on Suicidality—which Could Lead to Label Changes, Market Suspension or Market Withdrawal in 30 Nations
- Biotech Firm with Promising Treatment for Peripheral Nerve Damage Solicits Input from PFS PatientsWinSanTor’s topical pirenzepine 4% (WST-057) could be available for compassionate use in early 2025, says CEO Sept. 16, 2024 Dear Friends: If you’re among the many PFS patients whose symptoms include genital numbness—or numbness anywhere on your body—WinSanTor wants to hear from you pronto. Relief on the horizon? Earlier this month, the San Diego, Calif.-based… Read more: Biotech Firm with Promising Treatment for Peripheral Nerve Damage Solicits Input from PFS Patients
- 2024 PFS Foundation Annual AddressAug. 4, 2024 Dear Friends: Common sense would dictate that medical professionals are more likely than laymen to steer clear of risky pharmacological substances. They have, after all, read the product labels, and at least a smattering of the medical literature. They may also have witnessed adverse reactions in their own patients, and perhaps even… Read more: 2024 PFS Foundation Annual Address
- Brother of UK PFS Patient Who Took His Own Life Speaks out in the Wake of New Finasteride Warning‘We need to continually remind doctors that this drug could prove fatal to any patient,’ says Philip Dixon June 10, 2024 Dear Friends: The British are coming—to terms with the fact that most finasteride patients have no idea the drug can cause persistent sexual dysfunction and suicidal ideation. But in the heartbreaking case of Paul… Read more: Brother of UK PFS Patient Who Took His Own Life Speaks out in the Wake of New Finasteride Warning
- Team Melcangi Launches ‘Milano Project’ to Map the Basic Science of PFS so Research Can Move from an Animal Model to Human Clinical TrialsInitial therapeutic target is allopregnanolone April 11, 2024 Dear Friends: Roberto Cosimo Melcangi, PhD, is taking a page out of Robert Oppenheimer’s playbook. The Head of the Neuroendocrinology Unit in the Department of Pharmacological and Biomolecular Sciences at the University of Milano (UniMi) this week launched an initiative to supercharge post-finasteride syndrome (PFS) research and,… Read more: Team Melcangi Launches ‘Milano Project’ to Map the Basic Science of PFS so Research Can Move from an Animal Model to Human Clinical Trials
- Search for Sources of Major PFS Symptoms Pinpoints 186 Brain GenesNew UniMi study enumerates pathologies including depression, anxiety, insomnia, and cognitive dysfunction potentially linked to finasteride-induced genetic dysregulation March 16, 2024 Dear Friends: Turns out, post-finasteride syndrome may indeed be all in your head—namely, your hypothalamus and hippocampus. So demonstrates the latest research from the University of Milano (UniMi), which has identified 186 genes in… Read more: Search for Sources of Major PFS Symptoms Pinpoints 186 Brain Genes
- Canada to Health Pros: All Finasteride Patients Should ‘Be Screened for Suicidal Ideation, Self-harm, and Depression’ Before Being Prescribed the DrugJapanese disproportionality analysis of finasteride ADRs, meanwhile, shows completed suicides 270% higher than expected Jan. 28, 2024 Dear Friends: Canada is taking no chances vis-á-vis finasteride wreaking havoc on its citizens’ psyches. Pre-prescription edict Last week, drug-regulatory authority (DRA) Health Canada (HC), via its monthly Health Product InfoWatch bulletin, informed its nation’s healthcare professionals that:… Read more: Canada to Health Pros: All Finasteride Patients Should ‘Be Screened for Suicidal Ideation, Self-harm, and Depression’ Before Being Prescribed the Drug
- Internet Access to Prescription-Free Finasteride ‘Is a Serious Risk,’ Warns PFS Investigator Roberto Melcangi‘My colleagues are seeing many cases’ of this condition, the UniMi endocrinology professor notes in his first-ever long-form TV interview Jan. 7, 2024 Dear Friends: After a decade of being reduced to mere soundbites on TV news reports, Roberto Cosimo Melcangi is finally getting some quality time in the spotlight. Last month, Italy’s Byoblu TV—having… Read more: Internet Access to Prescription-Free Finasteride ‘Is a Serious Risk,’ Warns PFS Investigator Roberto Melcangi
- ED that Occurs During Finasteride Treatment Differs from ED that Persists Post-Treatment, Says New UniMi ResearchPFS Investigator Roberto Melcangi suspects the post-treatment variety springs from neuroendocrine disorder, rather than genital pathology Aug. 28, 2023 Dear Friends: Finasteride use can, and does, lead to erectile dysfunction that can, and does, last indefinitely in a subset of patients. By this point in the epidemiology of PFS, few self-respecting doctors would argue with… Read more: ED that Occurs During Finasteride Treatment Differs from ED that Persists Post-Treatment, Says New UniMi Research
- 51% of Dermatologists Believe Finasteride ‘May Cause’ Sexual Side Effects—but Only 18% Believe those ADRs May Persist, Says New Harvard Medical School ResearchPioneering PFS investigator Michael Irwig also finds that just 13% of respondents believe finasteride could cause depression, while 54% believe the drug is ‘unlikely’ or ‘very unlikely’ to do so Aug. 16, 2023 Dear Friends: Despite the fact that the finasteride product label has included depression and persistent loss of libido as possible side effects… Read more: 51% of Dermatologists Believe Finasteride ‘May Cause’ Sexual Side Effects—but Only 18% Believe those ADRs May Persist, Says New Harvard Medical School Research
- 2023 PFS Foundation Annual AddressAug. 4, 2023 Dear Friends: Finasteride was first approved by the US Food and Drug Administration in April 1993. But let’s put that aside for a moment. Let’s instead step back just a dozen years. In April 2011, I was driving down the Garden State Parkway, on my way from New York City, where I… Read more: 2023 PFS Foundation Annual Address
- Finasteride Manufacturer Organon Flees French Propecia Market, Rather than Comply with Federally Mandated Red-Box WarningBut generics, unfazed by front-facing disclosure that their products ‘can cause psychiatric and/or sexual disorders,’ stay put May 31, 2023 Dear Friends: French resistance to burying Propecia’s sexual and neuropsychiatric side effects apparently proved too much for the medication’s manufacturer. Last week, drug-regulatory authority ANSM issued a news update (English) noting that Organon & Co. (NYSE:… Read more: Finasteride Manufacturer Organon Flees French Propecia Market, Rather than Comply with Federally Mandated Red-Box Warning
- Neuropsychiatric Side Effects for Finasteride Housed on FDA Database Show Disproportionate Safety Signals Compared to Control Meds—and a ‘Striking Increase in Suicides’—Says New ResearchFollowing new safety review, Health Canada working with finasteride manufacturers to strengthen suicidality warning March 27, 2023 Dear Friends: Although the US Food and Drug Administration rarely mentions PFS, its database of side effects speaks volumes about the condition’s epidemiology. Out of control According to new research based in part on the agency’s Adverse Event… Read more: Neuropsychiatric Side Effects for Finasteride Housed on FDA Database Show Disproportionate Safety Signals Compared to Control Meds—and a ‘Striking Increase in Suicides’—Says New Research
- Study Used to Push Non-FDA-Approved Topical Finasteride Knocked by Top German Rx Journal‘It is still unclear to what extent the lower systemic drug exposure translates into a lower risk of side effects,’ says Deutsche Apotheker Zeitung. ‘The limited number of patients makes it difficult to draw reliable conclusions.’ March 20, 2023 Dear Friends: Germany’s oldest and most widely read pharmaceutical journal has taken a deep dive into… Read more: Study Used to Push Non-FDA-Approved Topical Finasteride Knocked by Top German Rx Journal
- UK Drug Regulatory Authority MHRA Investigating Finasteride SafetyProbe launched after tête-à-tête with French counterpart ANSM, whose ‘red box’ warning will be slapped on all finasteride 1 mg packaging next month March 5, 2023 Dear Friends: The British are coming—hopefully with a PFS-awareness program on par with that of the French. Dr. White does right According to a February 16 memo from Amelia… Read more: UK Drug Regulatory Authority MHRA Investigating Finasteride Safety
- Topical Finasteride Could Precipitate PFS, Top German Rx Journal WarnsStricken US college student corroborates red flag raised to 29,000 pharmacists Feb. 27, 2023 Dear Friends: Spraying finasteride on your scalp instead of ingesting it is no guarantee you won’t develop post-finasteride syndrome (PFS). So cautions Germany’s oldest and most widely read pharmaceutical journal. So, too, an American college senior who says his academic, social… Read more: Topical Finasteride Could Precipitate PFS, Top German Rx Journal Warns
- France Orders ‘Red-Box’ Warning—and QR Code Linking to PFS-Awareness Dossier—Slapped on all Finasteride 1 mg Products by AprilDec. 4, 2022 Dear Friends: French PFS awareness is going high-tech. ANSM, the Gallic nation’s drug-regulatory authority (DRA), last week unveiled plans to add a so-called “red-box” warning—that includes a QR code—on all finasteride 1 mg products in 2023. Intended to “reinforce information on adverse effects” of Propecia and generics, the warning reads: This medication… Read more: France Orders ‘Red-Box’ Warning—and QR Code Linking to PFS-Awareness Dossier—Slapped on all Finasteride 1 mg Products by April
- Why Is This Hair-loss Drug Still on the Market? German Public TV Asks of FinasterideReporter calls disclosure by nation’s drug-regulatory agency ‘shocking and unbelievable’ Nov. 2, 2022 Dear Friends: Anti-finasteride sentiment continues to run rife on Teutonic TV. Dürfen Die Das? (Can They Do That?), a newsmagazine show on the German public-broadcasting network NDR, last week debuted a 17-minute film titled Finasteride: Why Is This Hair-loss Drug Still on… Read more: Why Is This Hair-loss Drug Still on the Market? German Public TV Asks of Finasteride
- Allopregnanolone Counteracts Finasteride-induced Alterations in Gut Microbiota, According to New University of Milano ResearchStudy marks first-ever demonstration of a successful PFS therapy in an animal model Oct. 29, 2022 Dear Friends: The neurosteroid allopregnanolone (ALLO) has proved effective in counteracting some of the finasteride-induced alterations in gut microbiota, according to new research at the University of Milano (UniMi). Titled Gut Inflammation Induced by Finasteride Withdrawal: Therapeutic Effect of… Read more: Allopregnanolone Counteracts Finasteride-induced Alterations in Gut Microbiota, According to New University of Milano Research
- PFS Is ‘Clearly a Problem,’ ‘Warrants Intervention’ and Is ‘Inappropriate to Dismiss,’ Says New USC ResearchIsraeli pharmacovigilance expert, meanwhile, calls for ‘revolution’ in ADR reporting after local family of PFS suicide case files suit against Merck Sept. 26, 2022 Dear Friends: If you’ve ever Googled “post-finasteride syndrome,” chances are you’ve earned a place in the annals of medicine. Researchers at the University of Southern California’s Keck School of Medicine (KSM)… Read more: PFS Is ‘Clearly a Problem,’ ‘Warrants Intervention’ and Is ‘Inappropriate to Dismiss,’ Says New USC Research
- First-ever Suicidality ADRs Added to US Propecia Product Label, per FDA MandateBut Patient Product Information not revised to include the potentially lethal adverse events Aug. 29, 2022 Dear Friends: Organon & Co., the Merck & Co. spinoff that now owns Propecia (finasteride 1 mg), has added a new adverse drug reaction (ADR) to the hair-loss remedy’s label: suicidality. Earlier this month, Organon updated the Adverse Reactions… Read more: First-ever Suicidality ADRs Added to US Propecia Product Label, per FDA Mandate
- Family of Second Baylor-study Patient Who Took His Own Life Goes Public via New York Post ReportSteve Kenney (1972-2014) was a beloved detective protecting the people of DeKalb County, GA Aug. 13, 2022 Dear Friends: In Mohit Khera’s groundbreaking PFS research, published in June 2020, the Director for Andrology Research at Baylor College of Medicine wrote, “Two patients (8%) in the 5ARI arm committed suicide during or after the study period.”… Read more: Family of Second Baylor-study Patient Who Took His Own Life Goes Public via New York Post Report
- 2022 PFS Foundation Annual AddressAug. 4, 2022 Dear Friends: This month marks a full decade that we’ve been in the business of facilitating PFS research, generating awareness of the condition, providing support to patients suffering from the condition, and lobbying for stricter regulation so that fewer unsuspecting men develop the condition. Now, if at any time between August 2012… Read more: 2022 PFS Foundation Annual Address
- French Drug-Regulatory Authority Unveils World’s Most Thorough PFS-Prevention Program‘How-to’ video encourages reporting of finasteride ADRs to Ministry of Health July 13, 2022 Dear Friends: France’s National Agency for the Safety of Medicines and Health Products (ANSM) last week published a dossier of educational materials on the growing number of adverse drug reactions (ADRs) to finasteride 1 mg, as experienced by men currently suffering… Read more: French Drug-Regulatory Authority Unveils World’s Most Thorough PFS-Prevention Program
- New PFS Global Warning Map Plots When, Where and How Drug Regulatory Authorities Alerted Public to Finasteride’s Potential DangersJune 27, 2022 Dear Friends: The PFS Foundation has launched an interactive map plotting all the nations known to have issued warnings of finasteride’s potential to cause persistent adverse reactions, aka post-finasteride syndrome (PFS). Fifty-three nations, from Argentina to the United States, currently comprise our PFS Global Warning Map. Each links to a table entry… Read more: New PFS Global Warning Map Plots When, Where and How Drug Regulatory Authorities Alerted Public to Finasteride’s Potential Dangers
- Merck Warned Saudi Health Care Professionals in 2018 of Suicidal Ideation in Finasteride Patients, Confidential Document ShowsSeparately, safety signal of causal link between finasteride and diabetes mellitus issued by Saudi FDA in 2021 March 2, 2022 Dear Friends: In a confidential 2018 letter to health care professionals, Merck & Co.’s Saudi Arabian subsidiary warned that finasteride patients should be carefully monitored for psychiatric symptoms including anxiety, depression and suicidal ideation. Furthermore,… Read more: Merck Warned Saudi Health Care Professionals in 2018 of Suicidal Ideation in Finasteride Patients, Confidential Document Shows
- PFS Researcher Roberto Melcangi Featured in Alopecia-Treatment Documentary on Top Swiss TV NetJan. 22, 2022 Dear Friends: A new documentary examining three young men’s reactions to rapidly thinning locks—shaving, transplanting and finasteride—features an interview with eminent PFS researcher Roberto Cosimo Melcangi, PhD. Titled Plötzlich kahl (Suddenly Bald) the 30-minute film by Gustav Hofer (English-subtitled version here) debuted last month on SRF, the largest German-language broadcaster in Switzerland.… Read more: PFS Researcher Roberto Melcangi Featured in Alopecia-Treatment Documentary on Top Swiss TV Net
- Vigilant PFS Patient Prompts Action by UK’s FDA EquivalentJan. 10, 2022 Dear Friends: After enduring decades of symptoms that ran the gamut from tooth loss to testicular cancer, Ryan Clark prompted his nation’s drug agency to take a telemed to task for failing to adequately disclose finasteride’s dangers. And though he’d hoped for more significant action, the 54-year-old PFS patient from the northeast… Read more: Vigilant PFS Patient Prompts Action by UK’s FDA Equivalent
- First-Ever PFS Diagnostic Criteria Published in Medical LiteratureEditorial in leading journal, meanwhile, criticizes FDA for not making Merck conduct new safety studies when persistent ED signal emerged Nov. 20, 2021 Dear Friends: A decade after the first peer-reviewed article on persistent adverse sexual effects in finasteride patients appeared in medical literature, the first-ever diagnostic criteria for PFS has followed suit. We have… Read more: First-Ever PFS Diagnostic Criteria Published in Medical Literature
- PFS Foundation Sues FDA for Unlawfully Failing to Grant or Deny Our Citizen PetitionSept. 9, 2021 Dear Friends: The Post-Finasteride Syndrome Foundation, represented by consumer rights advocacy group Public Citizen, yesterday filed a lawsuit in Washington, DC, federal court compelling the US Food and Drug Administration to act on our Citizen Petition. Facts laid out in the eight-page complaint include: The “most serious risk of 1 mg finasteride… Read more: PFS Foundation Sues FDA for Unlawfully Failing to Grant or Deny Our Citizen Petition
- 2021 PFS Foundation Annual AddressAug. 4, 2021 Dear Friends: For several years now, our patient manager, Philip Roberts, has been telling me he feels like Radar O’Reilly on M*A*S*H. Fans of the dark Korean War comedy will recall that often after endless hours of stitching up battlefield casualties—and having just flopped onto their cots—the doctors would be interrupted by… Read more: 2021 PFS Foundation Annual Address
- Gene-Expression Study in the Penile Skin Tissue of PFS Patients Finds Significant Differences in the Expression of 3,764 GenesJuly 14, 2021 Dear Friends: The first study to consider and demonstrate gene expression differences as a potential etiology of the sexual dysfunction experienced by PFS patients has been published by a research team at Baylor College of Medicine (BCM). In all, the PFS patients had 1,446 significantly over-expressed (upregulated) and 2,318 significantly under-expressed (downregulated)… Read more: Gene-Expression Study in the Penile Skin Tissue of PFS Patients Finds Significant Differences in the Expression of 3,764 Genes
- Unsealed Documents from Propecia Litigation Now Housed on PFS Foundation WebsiteJune 29, 2021 Dear Friends: The PFS Foundation has launched a new section of its website to house documents from the US Propecia litigation unsealed by court order earlier this year. Part A of our Propecia Litigation Library (Paper Trail), contains 479 pages of such documents, along with 125 pages of related content, including Merck’s… Read more: Unsealed Documents from Propecia Litigation Now Housed on PFS Foundation Website
- In Addition to Blocking 5α-R, Finasteride Inhibits Adrenaline Production, Possibly Inducing Sexual and Psychological Side Effects, New Research SuggestsFinasteride patients face a 51% greater risk of suicidal ideation or suicidal behavior than the general population, separate study demonstrates April 13, 2021 Dear Friends: Phase V of the PFS Foundation-sponsored research at the University of Milano (UniMi) has found that finasteride, a 5-alpha reductase (5α-R) inhibitor, also inhibits phenylethanolamine N-methyltransferase (PNMT), an enzyme found… Read more: In Addition to Blocking 5α-R, Finasteride Inhibits Adrenaline Production, Possibly Inducing Sexual and Psychological Side Effects, New Research Suggests
- PFS Foundation Files Supplements to FDA Citizen Petition Seeking Finasteride’s Removal from the MarketUS District Court, meanwhile, orders all sealed documents in Propecia litigation to be unsealed February 1, 2021 Dear Friends: The PFS Foundation has filed supplements to its FDA Citizen Petition requesting that the agency “immediately require withdrawal of marketing approval for Propecia…because the risk of serious injury from the drug outweighs its limited benefits.” The… Read more: PFS Foundation Files Supplements to FDA Citizen Petition Seeking Finasteride’s Removal from the Market
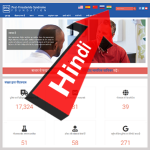
- New Hindi-Language Site Adds 615 Million Potential Readers to the PFS Awareness MovementJanuary 20, 2021 Dear Friends: The nation that gave us such wise figures as Mahatma Gandhi, Srinivasa Ramanujan and Anandibai Joshi just got a bit wiser. With respect to PFS, that is. Now, speakers of the world’s third most popular language can read all about post-finasteride syndrome (aka “पोस्ट-फ़ाइस्टरसाइड सिंड्रोम”) in their native tongue. To… Read more: New Hindi-Language Site Adds 615 Million Potential Readers to the PFS Awareness Movement
- Young Men Using Finasteride for Alopecia May Be More Suicide-Prone than the General Population, Says New Pharmacovigilance ResearchNov. 12, 2020 Dear Friends: Men 45 years old and younger who use finasteride for hair loss are three times more likely to experience suicidal ideation, plan their suicide or attempt suicide, according to a new pharmacovigilance investigation. Risk of such suicidality was 63 percent higher than among men using other alopecia medications, including minoxidil… Read more: Young Men Using Finasteride for Alopecia May Be More Suicide-Prone than the General Population, Says New Pharmacovigilance Research
- Gut Microbiota Population is Altered in PFS Patients, New Research DemonstratesSept. 28, 2020 Dear Friends: Phase IV of the PFS Foundation-sponsored research at the University of Milano (UniMi), designed to investigate the presence of altered gut microbiota in PFS patients, has successfully demonstrated the presence of altered gut microbiota in PFS patients. Titled Alterations of gut microbiota composition in post-finasteride patients: a pilot study, the… Read more: Gut Microbiota Population is Altered in PFS Patients, New Research Demonstrates
- 2020 PFS Foundation Annual AddressAug. 4, 2020 Dear Friends: Back in March, when COVID-19 first swept America, our patient manager was chatting with a thirtysomething patient who’s been suffering from PFS since 2010. Let’s call him Andrew. Andrew had recently run into a colleague from the last full-time job Andrew held before developing the condition and being forced to… Read more: 2020 PFS Foundation Annual Address
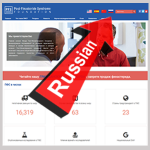
- New Russian-Language Website Boosts our Potential Readership by 265 MillionMay 6, 2020 Dear Friends: Thanks to the latest foreign-language edition of our website, PFS awareness is alive and well and living freely in Russia. Ditto those nations where Russian is an official or secondary language, including Armenia, Azerbaijan, Belarus, Estonia, Georgia, Israel, Kazakhstan, Kyrgyzstan, Latvia, Lithuania, Moldova, Mongolia, Tajikistan, Turkmenistan, Ukraine and Uzbekistan. Now,… Read more: New Russian-Language Website Boosts our Potential Readership by 265 Million
- Penile Vascular Abnormalities Found in Majority of PFS Patients in Baylor College of Medicine StudyApril 28, 2020 Dear Friends: In a newly published prospective case-control study in Translational Andrology and Urology, Mohit Khera, Director of the Laboratory for Andrology Research at Baylor College of Medicine, and his research team report the presence of vascular abnormalities in the penis of PFS patients (median age of 38) who previously discontinued the… Read more: Penile Vascular Abnormalities Found in Majority of PFS Patients in Baylor College of Medicine Study
- Wall Street Journal Letter to Editor Addresses Telemeds’ Easy Access to FinasterideMarch 11, 2020 Dear Friends: On February 21, The Wall Street Journal ran a 1,280-word story headlined Losing Your Hair? Why This Solution Is No Longer Shameful, which went, in part, like this: [In 1991]…effective methods like Rogaine or hair plugs were discussed in hushed tones. A… TV ad didn’t mention hair loss, hawking instead… Read more: Wall Street Journal Letter to Editor Addresses Telemeds’ Easy Access to Finasteride
- Young Men Who Use Finasteride for Hair Loss ‘Are at Risk for Suicide if They Develop Persistent Sexual Adverse Effects and Insomnia,’ Says New ResearchFebruary 20, 2020 Dear Friends: “Clinicians should be aware that men under the age of 40 who use finasteride for alopecia are at risk for suicide if they develop persistent sexual adverse effects and insomnia.” That, according to new research by Michael S. Irwig, MD, attending endocrinologist at Beth Israel Deaconess Medical Center in Boston,… Read more: Young Men Who Use Finasteride for Hair Loss ‘Are at Risk for Suicide if They Develop Persistent Sexual Adverse Effects and Insomnia,’ Says New Research
- France’s FDA Equivalent Issues Information Point and Fact Sheet on Finasteride Adverse EffectsDecember 19, 2019 Dear Friends: France’s FDA-equivalent agency last week issued an Information Point and fact sheet for men currently taking finasteride for hair loss, or for those considering taking the drug for hair loss. “Developed in conjunction with concerned patient associations and health professionals, this fact sheet aims to reinforce patient information on the… Read more: France’s FDA Equivalent Issues Information Point and Fact Sheet on Finasteride Adverse Effects
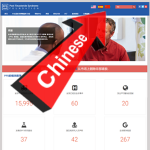
- New Chinese-Language Website Ups Our Potential Readership by 1+ BillionOctober 21, 2019 Dear Friends: PFS awareness took an enormous leap forward today—over the Great Wall and into the Sleeping Giant. At the stroke of midnight, we unveiled a Mandarin Chinese version of our website so that speakers of the world’s second most popular language can learn all about the condition (known to them as… Read more: New Chinese-Language Website Ups Our Potential Readership by 1+ Billion
- Reuters Report on Merck Hiding ‘Secrets’ about Propecia’s Risks Brings our FDA Citizen Petition to Light Ahead of Federal ProbeSept. 16, 2019 Dear Friends: Dan Levine, an investigative journalist with the largest news agency in the Western world, last week published a 3,900-word investigative report headlined Court let Merck hide secrets about a popular drug’s risks. A year in the making, the Reuters story uncovered testimony by former Merck executives in the US Propecia… Read more: Reuters Report on Merck Hiding ‘Secrets’ about Propecia’s Risks Brings our FDA Citizen Petition to Light Ahead of Federal Probe
- PFS Awareness Hops the Alps into ItalyAug. 17, 2019 Dear Friends: The Boot has begun kicking Propecia. Last week, Italian health and lifestyle magazine Starbene, which boasts a weekly circulation of 350,000, ran a story on PFS that begins: The entire world, including Italy, is experiencing a tragedy that requires our attention. It’s that of men, often young, who in hopes… Read more: PFS Awareness Hops the Alps into Italy
- 2019 PFS Foundation Annual AddressAug. 4, 2019 Dear Friends: Irrefutable. It’s a word we’re aiming to make synonymous with PFS by August 2022, when this foundation turns 10. That leaves us just 41 months. But if recent activity on the research and regulatory fronts are any indication—to say nothing of the surge in efforts by patients and their loved… Read more: 2019 PFS Foundation Annual Address
- Epigenetic Modifications Do Occur in PFS Patients, New Research DemonstratesJuly 20, 2019 Dear Friends: Phase III of the PFS Foundation-sponsored research at the University of Milano (UniMi), which was designed to “study whether epigenetic modifications occur in PFS patients,” has successfully demonstrated epigenetic modifications in PFS patients. Titled Altered methylation pattern of the SRD5A2 gene in cerebrospinal fluid of post-Finasteride patients, the pilot study… Read more: Epigenetic Modifications Do Occur in PFS Patients, New Research Demonstrates
- Evidence of Increased ED Rates in Finasteride Patients Is ‘More Compelling’ than Evidence Against, Says New Paper in JAADJune 5, 2019 Dear Friends: PFS naysayers’ days may be numbered. According to new research in the world’s most influential dermatology journal, medical science is leaning further toward the conclusion that finasteride can cause sexual dysfunction in men taking a 1-mg dose for hair loss. The paper, titled Sexual dysfunction in men taking systemic dermatologic… Read more: Evidence of Increased ED Rates in Finasteride Patients Is ‘More Compelling’ than Evidence Against, Says New Paper in JAAD
- Germany Joins French Defiance of FinasterideMay 30, 2019 Dear Friends: If PFS awareness is what it takes to unite Germany and France, so be it. Last week brought a slew of media coverage out of Deutschland that closely mirrors what we saw a month earlier in the French Republic. The awareness assault began May 21 with a report on the… Read more: Germany Joins French Defiance of Finasteride
- PFS Awareness Unfurling at a Furious Pace in FranceApril 29, 2019 Dear Friends: The French are not fond of finasteride. March brought a barrage of media coverage in the nation’s escalating efforts to warn citizens against the medication’s potential dangers. On the broadcast front, three network news programs highlighted the suffering of PFS patients. In a segment on the France 5 program Allo… Read more: PFS Awareness Unfurling at a Furious Pace in France
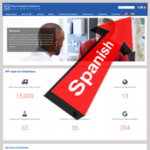
- New Spanish-Language Website Ups Our Potential Readership by 500+ MillionApril 11, 2019 Dear Friends: Today marks yet another milestone in PFS awareness: Spanish speakers across the globe can now read all about the condition—aka “síndrome post-finasterida” (SPF)—in their native tongue. To access our Spanish-language content, simply click the Spanish flag aside the search box at the top of any pfsfoundation.org page. To toggle back… Read more: New Spanish-Language Website Ups Our Potential Readership by 500+ Million
- Regulatory Update: Canada Concludes ‘There May Be a Link Between Finasteride and Risk of Suicidal Ideation’February 28, 2019 Dear Friends: Could Canada be thawing to the notion of a finasteride-free nation? Earlier this week, our neighbor to the north announced that Propecia and Proscar patients may be at risk for considering and attempting suicide, and mandated that the manufacturer update its product insert to reflect this potential danger. The move… Read more: Regulatory Update: Canada Concludes ‘There May Be a Link Between Finasteride and Risk of Suicidal Ideation’
- PFS is Front-page News in the NetherlandsFebruary 25, 2019 Dear Friends: PFS awareness is fanning out across Europe faster than a leaky dyke. The latest nation to take up our charge is the Netherlands, via its second-largest newspaper, De Volkskrant, which boasts a daily circulation of more than 250,000. In a 2,600-word story that ran last week, reporter Irene de Zwaan… Read more: PFS is Front-page News in the Netherlands
- French Urologist to Top TV News Mag: Propecia is a potentially dangerous drug—’I even told my brother-in-law to stop taking it’February 10, 2019 Dear Friends: Vive la France! This time we call your attention to a PFS report last week on 66 Minutes, which models itself after the iconic American news magazine 60 Minutes and boasts a Sunday-evening audience of two million viewers. The 14-minute segment, titled Is This Hair-loss Drug Dangerous? (English-subtitled version here),… Read more: French Urologist to Top TV News Mag: Propecia is a potentially dangerous drug—’I even told my brother-in-law to stop taking it’
- Regulatory Update: France’s FDA-Equivalent Agency Reissues Finasteride WarningFebruary 2, 2019 Dear Friends: Prompted by an increasing number of adverse drug-reaction reports to finasteride, France’s FDA-equivalent agency yesterday issued a letter to 100,000 health professionals reminding them that patients treated with the drug are at risk for developing psychiatric disorders and persistent sexual disorders. The Agence Nationale de Sécurité du Médicament et des… Read more: Regulatory Update: France’s FDA-Equivalent Agency Reissues Finasteride Warning
- Noted Swiss Science Writer Sides with Nation’s 2,000 PFS SufferersFebruary 1, 2019 Dear Friends: Looks like Switzerland’s not so neutral after all. At least when it comes to finasteride. In a SonntagsZeitung story headlined Finasteride was once considered a miracle cure for hair loss. But soon libido disorders and other serious side effects were discovered. Now it’s going to court (English translation here ),… Read more: Noted Swiss Science Writer Sides with Nation’s 2,000 PFS Sufferers
- France’s Le Monde to 300,000 Readers: Finasteride Users BewareJanuary 25, 2019 Dear Friends: France is at it again. Doing the right thing, that is. Earlier this week, Le Monde—the nation’s second-largest newspaper, with a daily circulation of more than 300,000 copies—ran a story headlined When Fighting Hair Loss, Watch out for Finasteride. (English translation here.) The 1,170-word report, by Pascale Santi, begins: “Romain… Read more: France’s Le Monde to 300,000 Readers: Finasteride Users Beware
- Finasteride ‘Causes Several Alterations’ in the Section of the Brain Responsible for Processing Long-term Memory and Emotional Responses, New Animal-model Study DemonstratesSOMERSET, N.J., Oct. 1, 2018 – “Finasteride treatment causes several alterations in the hippocampus,” the section of the brain responsible for processing long-term memory and emotional responses, according to a new study conducted at the University of Milano, and the Cajal Institute and Carlos III Health Institute, both in Madrid. Titled Treatment of male rats… Read more: Finasteride ‘Causes Several Alterations’ in the Section of the Brain Responsible for Processing Long-term Memory and Emotional Responses, New Animal-model Study Demonstrates
- New PFS Foundation Website Welcomes Increased Patient InvolvementSept. 7, 2018 Dear Friends: The new PFS Foundation website is finally here, and we couldn’t be more enthusiastic, because it promises to speed along numerous global-awareness and patient-support efforts. You’ll note that the homepage features an info-graphic called “PFS by the Numbers” that functions as a running tab of data in six categories: Adverse… Read more: New PFS Foundation Website Welcomes Increased Patient Involvement
- 2018 PFS Foundation Annual AddressAug. 4, 2018 Dear Friends: November 23 is a day I wish I could strike forever from the calendar. For it’s the anniversary of my son Randy’s death. And this coming November 23 will be particularly difficult, marking a decade since his passing. On that day in 2008, despite my degrees from Johns Hopkins and… Read more: 2018 PFS Foundation Annual Address
- France’s No. 1 News Magazine Takes Federal Drug Agency to Task for Letting PFS ProliferateJuly 31, 2018 Dear Friends: Thirty-thousand Frenchman can’t all be immune to PFS. And the nation’s most widely read news magazine seems bent on proving it. In a July 21 feature story headlined Finasteride, the Controversial Drug that Medical Authorities Continue to Defend (English translation here), investigative reporters Camélia Echchihab and Emre Sari write: “Can… Read more: France’s No. 1 News Magazine Takes Federal Drug Agency to Task for Letting PFS Proliferate
- Regulatory Update: Germany’s FDA Equivalent Issues ‘Red Hand Letter’ on Finasteride ADRsJuly 6, 2018 Dear Friends: Apparently heeding an appeal by Germany’s largest weekly newspaper, the Federal Institute for Drugs and Medical Devices (BfArM) has dispatched a so-called Red Hand Letter to doctors and pharmacists nationwide, informing them of severe and persistent adverse drug reactions (ADRs) to finasteride. Five months ago, we told you that Die… Read more: Regulatory Update: Germany’s FDA Equivalent Issues ‘Red Hand Letter’ on Finasteride ADRs
- ‘We Wouldn’t Recommend That Any Man Take’ Finasteride, Urologist at University Hospital Zurich Tells Swiss TV News MagazineMay 20, 2018 Dear Friends: Yet another European nation has taken to the airwaves with PFS awareness. This time it’s Switzerland. The network is Swiss Radio and Television (SRF). And the program is Puls. On May 7, the weekly health-news magazine debuted a special report titled Do Anti-Baldness Remedies Make You Impotent? (English-subtitled version here.)… Read more: ‘We Wouldn’t Recommend That Any Man Take’ Finasteride, Urologist at University Hospital Zurich Tells Swiss TV News Magazine
- Common Pathways Between PFS and Post-SSRI Sexual Dysfunction Could Be Useful in Designing Therapeutic Strategies for Both, Says New University of Milano StudyApril 30, 2018 Dear Friends: Roberto Cosimo Melcangi, Ph.D., head of the Neuroendocrinology Unit in the Department of Pharmacological and Biomolecular Sciences at the University of Milano, has published a new paper in the current issue of Endocrine: International Journal of Basic and Clinical Endocrinology. Titled Post-finasteride syndrome and post-SSRI sexual dysfunction: two sides of… Read more: Common Pathways Between PFS and Post-SSRI Sexual Dysfunction Could Be Useful in Designing Therapeutic Strategies for Both, Says New University of Milano Study
- Regulatory Update: ‘Muscle-related Disorders’ Added to Canadian Finasteride Label in Response to Report by FDA-equivalent AgencyJuly 28, 2018 Dear Friends: Canada has taken an important first step toward keeping its citizens apprised of finasteride’s many potentially serious and persistent side effects. In the June edition of its Health Product InfoWatch, Health Canada (Canada’s version of the US Food and Drug Administration) reported that “New information regarding the risk of muscle-related… Read more: Regulatory Update: ‘Muscle-related Disorders’ Added to Canadian Finasteride Label in Response to Report by FDA-equivalent Agency
- Leading German Paper Calls on Federal Institute for Drugs to Better Educate Doctors, Patients on PFSMarch 12, 2018 Dear Friends: PFS is a growing concern in Germany, too. Ditto Albania. In December, we told you that the French National Agency for Drug Safety (ANSM) launched a public-awareness campaign warning against reported mood changes in Propecia patients, “particularly depression, and suicidal ideation.” Now we’re encouraged to report that Die Zeit, Germany’s… Read more: Leading German Paper Calls on Federal Institute for Drugs to Better Educate Doctors, Patients on PFS
- Possible Epigenetic Changes in PFS Patients Is Focus of New Clinical StudySOMERSET, N.J., Jan. 29, 2018 – The Post-Finasteride Syndrome Foundation today announced Phase II of the clinical research on post-finasteride syndrome (PFS) being conducted at the University of Milano. Led by Roberto Cosimo Melcangi, Ph.D., head of the Neuroendocrinology Unit in the Department of Pharmacological and Biomolecular Sciences, the new study will evaluate: —In PFS… Read more: Possible Epigenetic Changes in PFS Patients Is Focus of New Clinical Study
- French Warning of Propecia-Induced Depression and Suicidal Ideation Prompts Wave of Media AttentionDec. 19, 2017 Dear Friends: France is taking PFS very seriously. On Oct. 26, the French National Agency for Drug Safety (ANSM) issued a warning that “Changes in mood, particularly depression, and suicidal ideation, have been reported by patients taking Propecia.” The agency—France’s equivalent of the US Food and Drug Administration—noted that “finasteride treatment should… Read more: French Warning of Propecia-Induced Depression and Suicidal Ideation Prompts Wave of Media Attention
- Regulatory Update: European Medicines Agency Recommends Adding Depression and Suicidal Ideation to Finasteride LabelAug. 10, 2017 Dear Friends: On August 4, CEO John Santmann wrote in his 2017 Annual Address, “Recent events in Europe and Asia have given me hope that, before this decade is out, we’ll see the day when no man on Earth is prescribed finasteride without ample warning of its many potential dangers.” Then he… Read more: Regulatory Update: European Medicines Agency Recommends Adding Depression and Suicidal Ideation to Finasteride Label
- 2017 PFS Foundation Annual AddressAug. 4, 2017 Dear Friends: Recent events in Europe and Asia have given me hope that, before this decade is out, we’ll see the day when no man on earth is prescribed finasteride without ample warning of its many potential dangers. On May 24, the UK’s equivalent of the US Food and Drug Administration, the… Read more: 2017 PFS Foundation Annual Address
- Regulatory Update: Korea Mandates Propecia Label Change Based on Reports of Depression and Suicidal IdeationJuly 15, 2017 Dear Friends: On July 4, the Korean Ministry of Food and Drug Safety (MFDS) announced it will revise the warning label on Propecia and its generic versions to include depression and suicidal ideation. The move by South Korea’s equivalent of the US Food and Drug Administration came just six weeks after the… Read more: Regulatory Update: Korea Mandates Propecia Label Change Based on Reports of Depression and Suicidal Ideation
- Regulatory Update: MHRA Issues Drug-Safety Update on FinasterideMay 26, 2017 Dear Friends: The UK’s equivalent of the US Food and Drug Administration, the Medicines and Healthcare Products Regulatory Agency (MHRA), has issued a drug safety update on finasteride. The May 24 document, titled Finasteride: rare reports of depression and suicidal thoughts, states: Some men have reported episodes of depressive illness in association with… Read more: Regulatory Update: MHRA Issues Drug-Safety Update on Finasteride
- Peripheral Nervous System Involved in PFS Patients with Severe ED, New Study DemonstratesSOMERSET, N.J., April 18, 2017 – Post-finasteride syndrome (PFS) patients suffer from altered levels of critical brain-function regulators, including neuroactive steroids, according to a new clinical study published in The Journal of Steroid Biochemistry and Molecular Biology. Titled Neuroactive Steroid Levels and Psychiatric and Andrological Features in Post-Finasteride Patients, the three-year study also uncovered evidence… Read more: Peripheral Nervous System Involved in PFS Patients with Severe ED, New Study Demonstrates
- Feinberg School of Medicine Epidemiology Study Suggests Tens of Thousands of PFS Cases in Young Men Taking Finasteride for Hair LossSOMERSET, N.J., March 9, 2017 – More than one percent of young men who took finasteride for 206 days or longer developed persistent erectile dysfunction (PED) that lasted an average of 4.2 years after drug discontinuation, according to a new study published today in PeerJ. In all, researchers examined the electronic medical records (EMR) of… Read more: Feinberg School of Medicine Epidemiology Study Suggests Tens of Thousands of PFS Cases in Young Men Taking Finasteride for Hair Loss
- ‘Underlying Neurobiological Abnormalities’ Exist in Finasteride Users with Persistent Sexual Dysfunction, Suggests Clinical StudySOMERSET, N.J., Sept. 29, 2016 – Men who experience persistent sexual dysfunction after discontinuing finasteride have “neurobiological abnormalities,” suggests a new study published in The Journal of Clinical Endocrinology & Metabolism. Among those abnormalities is neural circuitry that overlaps with functional abnormalities identified in men suffering from “major depression.” Titled Characteristics of Men Who Report… Read more: ‘Underlying Neurobiological Abnormalities’ Exist in Finasteride Users with Persistent Sexual Dysfunction, Suggests Clinical Study
- Support Program Connects PFS Patients WorldwideSOMERSET, N.J., Sept. 21, 2016 – The Post-Finasteride Syndrome Foundation has launched a program to connect PFS patients with one another for mutual support. Any PFS patient or family member of a PFS patient who would like to participate should download the PFS Patient Support form, complete it and email it back to social@pfsfoundation.org. Due… Read more: Support Program Connects PFS Patients Worldwide
- 2016 PFS Foundation Annual AddressAug. 4, 2016 Dear Friends: I began last year’s annual address by letting you know how encouraged I was that the National Institutes of Health had not only added PFS to its Genetic and Rare Diseases Information Center but linked to our website. Now I’m equally encouraged to note a similar milestone, achieved exactly one… Read more: 2016 PFS Foundation Annual Address
- Two European Documentaries a Milestone in PFS AwarenessSOMERSET, N.J., July 14, 2016 – A pair of European documentaries focusing on post-finasteride syndrome mark the first time the condition has been the subject of extended TV coverage on any continent. The first documentary, titled “Pills in Search of an Illness,” debuted April 19 on the Spanish public TV network TV3 in Barcelona. Reported… Read more: Two European Documentaries a Milestone in PFS Awareness
- Finasteride-Induced Suicides up 8.4% in WHO’s VigiBaseSOMERSET, N.J., July 13, 2016 – Reports of finasteride-induced completed suicides are up 8.4% in the World Health Organization Progamme for International Drug Monitoring’s database of adverse drug reactions (ADRs) for Q1 through Q2 2016 over Q4 2015. The absolute number of completed suicides rose from 59 to 64 within that period. Also in the… Read more: Finasteride-Induced Suicides up 8.4% in WHO’s VigiBase
- Finasteride-Induced Suicidal and Self-Injurious Behavior Rises 33% in WHO Database of Adverse Drug ReactionsSOMERSET, N.J., Jan. 5, 2016 – Reports of finasteride-induced Suicidal and Self-Injurious Behavior are up 33 percent in the World Health Organization Progamme for International Drug Monitoring’s database of adverse drug reactions (ADRs) for Q4 vs. Q3 2015. Within that category (itself a subcategory of Psychiatric Disorders), suicidal ideaton is up 47.2%; suicide attempts up… Read more: Finasteride-Induced Suicidal and Self-Injurious Behavior Rises 33% in WHO Database of Adverse Drug Reactions
- PFS Foundation Web Traffic Up 33% Year Over YearSOMERSET, N.J., Jan. 5, 2016 – Traffic to the PFS Foundation website rose 33.6 percent in 2015, compared to 2014, according to Google Analytics. Concurrently, users in 161 countries—or 82.5 percent of the world (as defined by the 195 nations recognized by the U.S. Department of State)—accessed information on the condition via PFSFoundation.org in 2015,… Read more: PFS Foundation Web Traffic Up 33% Year Over Year
- Reminder: Report PFS Symptoms to National Pharmacovigilance AuthoritiesSOMERSET, N.J., Sept. 9, 2015 – The Post-Finasteride Syndrome Foundation is reminding PFS patients to report all persistent side effects to their respective national pharmacovigilance authorities for inclusion in the World Health Organization Progamme for International Drug Monitoring’s VigiBase database for adverse drug reactions (ADRs). Run by Uppsala Monitoring Centre (UMC), which provides scientific leadership… Read more: Reminder: Report PFS Symptoms to National Pharmacovigilance Authorities
- Data Available on Adverse Side Effects of 5α-RIs Don’t Conclusively Suggest They’re Safe, Say Researchers at Boston University School of MedicineSOMERSET, N.J., Sept. 7, 2015 – Data from preclinical and clinical studies have provided substantial evidence that 5α-Reductase inhibitors (5α-RIs) such as finasteride and dutasteride cause loss or reduction of libido, increase the risk of erectile dysfunction and ejaculatory dysfunction, and may contribute to the onset of depression. These agents interfere with the biosynthesis and… Read more: Data Available on Adverse Side Effects of 5α-RIs Don’t Conclusively Suggest They’re Safe, Say Researchers at Boston University School of Medicine
- Suggestion That Sexual Side Effects Appear Early in Finasteride Therapy then Return to Normal is ‘Inaccurate,’ Say Researchers at Boston University School of MedicineSOMERSET, N.J., Aug. 26, 2015 – Twenty-five percent of men currently taking finasteride or dutasteride (brand names Proscar and Avodart) for the treatment of benign prostate enlargement (BPH), appear not to benefit from taking these medications. Those prescribed Propecia or Avodart for male pattern hair loss are also at risk for adverse events elicited by… Read more: Suggestion That Sexual Side Effects Appear Early in Finasteride Therapy then Return to Normal is ‘Inaccurate,’ Say Researchers at Boston University School of Medicine
- 2015 PFS Foundation Annual AddressAug. 4, 2015 Dear Friends: By many accounts, we’ve reached a tipping point on post-finasteride syndrome awareness within the medical community. The most significant advancement occurred in March, when the U.S. National Institutes of Health added PFS to its Genetic and Rare Diseases Information Center. “Studies are under way to understand the safety profile of… Read more: 2015 PFS Foundation Annual Address
- Suicidal Ideation Associated with Finasteride Use in Young Men, Says New Study in Leading Pharmacology JournalSOMERSET, N.J., July 23, 2015 – New research in a leading pharmacology journal concludes that young men who take finasteride for hair loss may be at risk of contemplating suicide. The study, published in the July issue of Pharmacotherapy and titled “Persistent Sexual Dysfunction and Suicidal Ideation in Young Men Treated with Low-Dose Finasteride,” analyzed… Read more: Suicidal Ideation Associated with Finasteride Use in Young Men, Says New Study in Leading Pharmacology Journal
- Southwest Brain Bank Launches PFS Brain and Spinal Cord Donation ProgramSOMERSET, N.J., July 15, 2015 – The Southwest Brain Bank (SWBB) in the Department of Psychiatry at the University of Texas Health Science Center in San Antonio has received its first donation of the brain and spinal cord of a post-finasteride syndrome (PFS) patient and formed a program to collect and study post-mortem human brain… Read more: Southwest Brain Bank Launches PFS Brain and Spinal Cord Donation Program
- U.S. National Institutes of Health Adds PFS to Genetic and Rare Diseases Info CenterMay 19, 2015 Dear Public Health Official: The U.S. National Institutes of Health recently added post-finasteride syndrome (PFS) to its Genetic and Rare Diseases Information Center, noting that: “Studies are underway to understand the safety profile of 5-alpha reductase inhibitor drugs with respect to adverse events…and their permanency.” This federal listing of PFS comes in… Read more: U.S. National Institutes of Health Adds PFS to Genetic and Rare Diseases Info Center
- Number of Nations Logging onto PFS Foundation Website Rises 22% in 2014SOMERSET, N.J., Jan. 6, 2015 – The number of nations that logged onto the Post-Finasteride Syndrome Foundation website jumped 22 percent in 2014, according to Google Analytics. In all, users in 157 countries—or 81 percent of the world (as defined by the 195 nations recognized by the U.S. Department of State)—accessed information on the condition… Read more: Number of Nations Logging onto PFS Foundation Website Rises 22% in 2014
- Clinical Study of Post-Finasteride Syndrome Launched at University of Milano-Bicocca and University of MilanoSOMERSET, N.J., Dec. 9, 2014 – The Post-Finasteride Syndrome Foundation today announced the funding of a third clinical study on post-finasteride syndrome (PFS), this one a collaboration between the University of Milano-Bicocca and the University of Milano, both in Italy. Titled “Rare, but Serious and Persistent, Side Effects of 5α Reductase Inhibitors (5ARI): Why Do… Read more: Clinical Study of Post-Finasteride Syndrome Launched at University of Milano-Bicocca and University of Milano
- 2014 PFS Foundation Annual AddressAug. 4, 2014 Dear Friends: I’m pleased to report that 2014 is shaping up to be a turning point on the road to widespread awareness of post-finasteride syndrome. Couple that with the steady progress of our clinical studies and I become ever more hopeful that the coming year will bring not only worldwide acceptance of… Read more: 2014 PFS Foundation Annual Address
- Number of Nations Logging onto PFS Foundation Website Rises Another 26% in First Half of 2014SOMERSET, N.J., July 8, 2014 – The number of nations that logged onto the Post-Finasteride Syndrome Foundation website rose 26 percent during the first half of 2014, according to Google Analytics. In all, users in 147 countries—or 75 percent of the world (as defined by the 195 nations recognized by the U.S. Department of State)—accessed… Read more: Number of Nations Logging onto PFS Foundation Website Rises Another 26% in First Half of 2014
- Doctors Recruiting PFS Patients for Research StudiesSOMERSET, N.J., June 16, 2014 – Post-finasteride syndrome research initiatives at two major U.S. medical institutions, Brigham and Women’s Hospital (a Harvard Medical School Teaching Affiliate) in Boston, Mass., and Baylor College of Medicine in Houston, Texas, are currently recruiting patients for their respective studies on the condition. If you are interested in participating, or… Read more: Doctors Recruiting PFS Patients for Research Studies
- Daniel M. Stewart: 1976-2014April 25, 2014 Dear Friends: The Post-Finasteride Syndrome Foundation is saddened to announce the passing of Daniel M. Stewart. The 37-year-old native of Troy, Michigan, and Centertown, Kentucky, died Saturday, April 12, 2014 at his home in Denton, Texas. Daniel, who suffered from post-finasteride syndrome, had recently taken part in a clinical-research study on the… Read more: Daniel M. Stewart: 1976-2014
- Number of Nations Logging onto PFSFoundation.org Rose 29% in 2013SOMERSET, N.J., Jan. 6, 2014 – The number of nations that logged onto the Post-Finasteride Syndrome Foundation website rose 29 percent in 2013, according to Google Analytics. In all, users in 120 countries outside the United States—or 61 percent of the world (as defined by the 195 nations recognized by the U.S. Department of State)—accessed… Read more: Number of Nations Logging onto PFSFoundation.org Rose 29% in 2013
- Reminder: Report PFS Symptoms to FDA MedWatchSOMERSET, N.J., Oct. 29, 2013 – The Post-Finasteride Syndrome Foundation is reminding PFS patients to report all persistent side effects to the FDA MedWatch program. MedWatch is the U.S. Food and Drug Administration’s system for detecting safety-hazard signals for pharmaceutical products and medical devices. If a signal is detected, the FDA can issue safety alerts,… Read more: Reminder: Report PFS Symptoms to FDA MedWatch
- Clinical Study of Post-Finasteride Syndrome Launched at Baylor College of MedicineSOMERSET, N.J., Aug. 21, 2013 – The Post-Finasteride Syndrome Foundation today announced the funding of a second major clinical study on post-finasteride syndrome (PFS), this one at Baylor College of Medicine (BCM) in Houston, Texas. Titled “Genetic and Epigenetic Studies on Post-Finasteride Syndrome Patients,” the research is being led by Mohit Khera (right), MD, MBA,… Read more: Clinical Study of Post-Finasteride Syndrome Launched at Baylor College of Medicine
- PFS Patient Query: Suicide CasesAugust 7, 2013 Dear Friends: The PFS Foundation is seeking information about patients who have taken finasteride or dutasteride, and who have attempted suicide or who have completed suicide. This is an area of active research interest and researchers are seeking to obtain medical records and other relevant material for such patients. To… Read more: PFS Patient Query: Suicide Cases