December 19, 2019
Dear Friends:
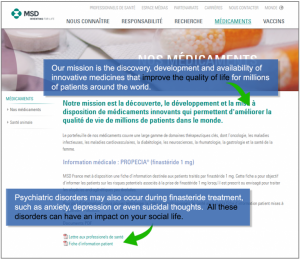 France’s FDA-equivalent agency last week issued an Information Point and fact sheet for men currently taking finasteride for hair loss, or for those considering taking the drug for hair loss.
France’s FDA-equivalent agency last week issued an Information Point and fact sheet for men currently taking finasteride for hair loss, or for those considering taking the drug for hair loss.
“Developed in conjunction with concerned patient associations and health professionals, this fact sheet aims to reinforce patient information on the risk of certain adverse effects, such as psychiatric disorders and/or sexual dysfunction, associated with finasteride use,” said The Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM) (English translation here).
“Use of finasteride 1 mg is currently being closely monitored at both the European and national levels,” the agency added. “It is important that information about its safety is shared widely. To that end, in October 2019, ANSM met with patient representatives and health professionals to develop an information document that supplements the leaflet contained in boxes of finasteride 1 mg.”
In a letter dated November 2019 (English translation here), ANSM has required MSD France , a subsidiary of finasteride manufacturer Merck & Co., to send the fact sheet (English translation here) to dermatologists, general practitioners and pharmacists with the intention that it be given to patients by doctors when Propecia is first prescribed, and by pharmacists to patients when the drug is dispensed.
Among its messages are:
- Sexual Disorders: “Patients have reported sexual disorders including erectile dysfunction, ejaculatory dysfunction, testicular pain and decreased libido. These effects may persist after stopping treatment for an indefinite period.”
- Psychiatric Disorders: “Psychiatric disorders may also occur during finasteride treatment, such as anxiety, depression or even suicidal thoughts. All these disorders can have an impact on your social life.”
- How Long Do Adverse Effects Last? “The time it takes for adverse events to set in during finasteride treatment may vary, from a few days to a few years after starting treatment. The duration of the adverse effects may also vary widely from patient to patient. Adverse effects may persist after stopping treatment, and in some cases for an indefinite period.”
 Anyone living in the US who suffers from PFS should report his symptoms to the US Food and Drug Administration. Anyone living outside the US who suffers from PFS should report his symptoms to the US Food and Drug Administration as well as to his national drug-regulatory agency, as directed on our Report Your Side Effects page.
Anyone living in the US who suffers from PFS should report his symptoms to the US Food and Drug Administration. Anyone living outside the US who suffers from PFS should report his symptoms to the US Food and Drug Administration as well as to his national drug-regulatory agency, as directed on our Report Your Side Effects page.
Related News
Regulatory Update: France’s FDA-Equivalent Agency Reissues Finasteride Warning
Regulatory Update: Germany’s FDA Equivalent Issues ‘Red Hand Letter’ on Finasteride ADRs
Regulatory Update: MHRA Issues Drug Safety Update on Finasteride
