‘It is still unclear to what extent the lower systemic drug exposure translates into a lower risk of side effects,’ says Deutsche Apotheker Zeitung. ‘The limited number of patients makes it difficult to draw reliable conclusions.’
March 20, 2023
Dear Friends:
Germany’s oldest and most widely read pharmaceutical journal has taken a deep dive into post-finasteride syndrome (PFS).
 That, in an effort to provide its 29,000 subscribers with the most scientifically sound information on non-FDA-approved topical finasteride.
That, in an effort to provide its 29,000 subscribers with the most scientifically sound information on non-FDA-approved topical finasteride.
That, as well, ahead of yet another nation bringing topical finasteride to market as a “safer” alternative to the oral formulation.
Tomorrow, Korean pharmaceutical firm Boryung (003850.KS)—with the blessing of the Ministry of Food and Drug Safety—will launch Finjuve finasteride spray in the Republic of Korea. Manufactured by UK-based Hikma Pharmaceuticals (LSE: HIK), Finjuve is already approved in Germany, Italy, Portugal and Luxembourg.
Due diligence
Titled Side Effects: Hair Growth with Consequences (English abstract) the 2,000-word meta-analysis by Tony Daubitz, PhD, was published March 10 in Deutsche Apotheker Zeitung (DAZ), just one month after its first report on the issue: Is Topical Finasteride a Better Alternative to Treat Alopecia?(English).
In all, Dr. Daubitz, who earned his doctorate at the Max Delbrück Center for Molecular Medicine in Berlin, cites from 28 clinical studies, many of whose authors are no doubt familiar to readers of this newsfeed, including:
Roberto Cosimo Melcangi, PhD, of the University of Milano, Mohit Khera, MD, of Baylor College of Medicine, Michael S. Irwig, MD, of Harvard Medical School, and Abdulmaged M. Traish, PhD, of Boston University School of Medicine.
He also provides the chart below of 17 PFS symptoms from published case reports:
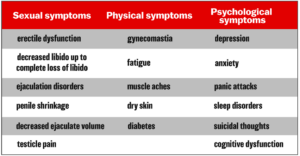 After laying out evidence supporting the hypothesis that a proportion of all patients taking oral finasteride develop PFS, Dr. Daubitz writes:
After laying out evidence supporting the hypothesis that a proportion of all patients taking oral finasteride develop PFS, Dr. Daubitz writes:
A new treatment alternative [to oral finasteride] is topical finasteride (Fynzur, 2275 mg/ml), which was recently launched in Germany. In a phase III study with 250 volunteers [Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia], the spray significantly stimulated hair growth…and showed similar effects to oral finasteride… On the other hand, the systemic drug exposure with dermal application was less than one hundredth of that with oral application. The DHT levels in the blood also fell correspondingly less…which is why fewer side effects are expected.
Proceed with extreme caution
So topical finasteride is safer, right? End of story, right? Hardly, writes Dr. Daubitz:
What sounds promising at first glance appears in a different light through an analysis of the drug. It is not possible to say whether the effect of the topical solution is really equivalent to that of the tablet, since the study was not designed to statistically evaluate this endpoint. And that’s just the beginning of its shortcomings. Only 71% of the subjects completed the study at all, and only 55% of the subjects had evaluable photos to assess the success of the therapy.
In a responder analysis commissioned by the [Federal Institute for Drugs and Medical Devices], the subjects were also asked to rate the improvement in their hair growth themselves. However, there were no significant differences in the subjective assessment (improvement in hair growth in 39.8% of men with topical finasteride, 31.0% with oral finasteride and 32.0% with placebo).
Then he concludes:
It is still unclear to what extent the lower systemic drug exposure translates into a lower risk of side effects. Although sexual side effects were not reported more frequently than with placebo… the limited number of patients in the study, as noted by the Arznei-Telegramm, makes it difficult to draw reliable conclusions. Nevertheless, it is important to anticipate the occurrence of such side effects.
Hims’ growing infamy
Before Dr. Daubitz signs off, however, he notes:
Although topical finasteride is not approved as a medicinal product in the USA, it is marketed as an over-the-counter medicinal product through telemedicine portals such as ForHims.com. When asked by the DAZ editors about this, the Post-Finasteride Syndrome Foundation points out that they are aware of numerous cases of the condition resulting from topical finasteride application. Further research on the syndrome is therefore urgently needed.
Truer words were never typed. Last month we revealed that, to date, 12 PFS patients have filed case reports with us indicating that they developed the condition after using topical finasteride only (Topical Finasteride Could Precipitate PFS, Top German Rx Journal Warns).
The first case to go public was that of Sumair Ahluwalia, a 21-year-old college student from Chicago, who developed full-blown PFS shortly after being prescribed Topical Finasteride & Minoxidil Spray by Hims nurse practioner Charlene Fernandez.
Dr. Daubitz’s reference to Hims & Hers Health (NYSE: HIMS) marked the second time in as many months that the San Francisco-based telemed, which also markets oral and topical finasteride in the UK, was singled out in European media reports.
On March 4, The Daily Mail ran a story headlined Watchdog launches investigation into hair loss pill as men report huge rise in side effects including depression, low libido and erectile dysfunction. Its subhead read: “Figures show side effects of hair loss drug sold under brands like Hims rising.”
 For us, the increasing incidence of finasteride patients who report developing PFS after being prescribed the drug by Hims, or other telemeds, is a data point we’ve been chronicling for five years. As we noted in our 2018 Annual Address:
For us, the increasing incidence of finasteride patients who report developing PFS after being prescribed the drug by Hims, or other telemeds, is a data point we’ve been chronicling for five years. As we noted in our 2018 Annual Address:
Keshav Narain, an ophthalmology surgeon in San Jose, CA, wrote us: “I recently saw a patient on finasteride.… He obtained it without a prescription, through Hims. I believe that the vast majority of these individuals have no idea of the potential dangers of this medication. Aside from the known side effects that result from androgen blockade, we believe that a subset of patients end up with neurological effects.”
Eighteen months later, in March 2020, we wrote on the Opinion page of The Wall Street Journal:
We get an average of 45 new PFS patients a month banging on our digital door from across the globe, up 462% since 2018… An ever-growing number of these patients inform us that they were prescribed finasteride, virtually, by Hims, Keeps or Roman. And virtually without warning of PFS.
And just seven months ago, in a report on this newsfeed headlined First-ever Suicidality ADRs Added to US Propecia Product Label, per FDA Mandate, we wrote:
More problematic—in terms of ensuring that all US patients taking finasteride 1 mg or considering taking finasteride 1 mg are immediately informed that the FDA has acknowledged that the drug could precipitate suicide—are the Big 3 telemedicine services whose core businesses are at least partly dominated by finasteride sales.
Since their foundings in 2017, Hims, Keeps, and Ro (formerly Roman) have raised about $1.3 billion in venture capital…giving them ample means to aggressively target men looking for hair-loss treatments. The problem is, according to a review by the PFS Foundation of all three telemeds’ consumer digital content, not one of them [mentions the FDA-mandated] suicidality ADRs.
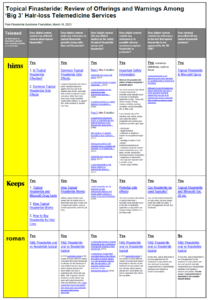 Ro v. wading into unregulated waters
Ro v. wading into unregulated waters
Now, with more reports coming our way of PFS patients developing the condition from topical finasteride only, we’ve produced a supplement to that research: Topical Finasteride: Review of Offerings and Warnings Among ‘Big 3’ Hair-loss Telemedicine Services.
The most salient observation is that, unlike competitors Hims and Keeps, Ro has opted not to prescribe/sell topical-finasteride products. Its rationale is simple:
At this time, topical finasteride is not FDA-approved for the treatment of male pattern hair loss and must be specially compounded by a compounding pharmacy and requires a doctor’s prescription. For this reason, compounded topical finasteride is not available through Roman.
Keeps, meanwhile, cannot seem to make up its mind as to whether topical finasteride is better or worse than oral finasteride.
On one hand, the sole product on its homepage is Topical Finasteride and Minoxidil Gel, which is thusly promoted: “Keeping your hair has never been easier. Get two clinically-proven products in a once-daily gel.”
On the other hand, its Topical Finasteride and Minoxidil Drug Facts page is such in name only. Apart from the term “topical finaseride” in its title, the page makes zero references to topical finasteride. And the two finasteride-related clinical studies cited on that page were published in 1998 and 1999, more than a decade before topical finasteride first appeared in medical literature as a possible therapy for androgenic alopecia (AGA).
Odder still, another Keeps page, How to Buy Finasteride for Hair Loss, contains a section titled, “Can finasteride be used topically?” which reads, in part:
You can find topical finasteride for sale online, but you should know that unlike the tablet, it isn’t FDA-approved for hair loss treatment… If more positive studies come to light, the FDA may consider changing its stance on topical finasteride. Until then, we’d suggest sticking with the oral version, just to be on the safe side.
So the pill it is, right? Not so fast.
Any would-be finasteride patient having the wherewithall to seek out the drug’s FDA-approved Prescribing Information (PI) will find a link to it on Keeps’ Finasteride page. The problem is, since we ran our Review of US FDA-mandated Suicidality ADR Disclosure five months ago, Keeps updated that page with the latest version of the PI, the one that now lists “suicidal ideation and behavior” as an adverse reaction.
It’s difficult to imagine an adverse reaction worse that suicide. But from the “logic” of Keeps’ digital consumer content, there could be one lurking in every bottle of its Topical Finasteride and Minoxidil Gel.
As for Hims, we got a firsthand look into its topical-finasteride prescription process last month. It’s worth adding here, however, that the $2 billion company promotes Topical Finasteride & Minoxidil Spray as its “#1 customer favorite” and “Most popular” product.
Since the FDA does not comment on products under consideration for marketing approval (or not), we sifted through the agency’s website for clues as to the future status of topical finasteride in the US. Policing-wise, we found two cases:
• Hybrid Pharma LLC: A 2021 inspection at this Deerfield Beach, Fla., outsourcing facility (aka compounding facility) yielded the following observation:
Your outsourcing facility did not submit a report to FDA upon initial registration as an outsourcing facility identifying the drugs compounded during the previous six-month period. Specifically, the following products were compounded and not identified on your reports: minoxidil 5%, tretinoin 0.01 %, finasteride 2.5% topical solution…
• Rx Unlimited Pharmacy: A 2022 inspection at this North Hills, Calif., outsourcing facility yielded the following observation:
Since May 2022, your company produced [REDACTED] drug product formulations (nasal spray, oral suspension, oral and topical solutions) using [REDACTED] as an ingredient. In the last three months your company dispensed [REDACTED] patient prescriptions with [REDACTED] as an ingredient. For example you dispensed the following compounded drug products:
—Finasteride Foam 0.2%
—Minoxidil 6%/Finasteride 0.2% Topical Solution
So we know that the FDA is paying attention to the production and dispensing of topical-finasteride products. But according to a report last updated March 14, 2023, finasteride is currently under evaluation as a “bulk drug substance” for use in compounding.
When Roman Kulikov learned that topical finasteride had been approved in Germany and Korea, he immediately stepped up to speak out on behalf of PFS patients worldwide.
“We were duped with the introduction of Propecia pills back in 1997, and now, 26 years later, we’re being duped again with these finasteride creams, gels and sprays,” says the 32-year-old cook from Berlin who has suffered from PFS since 2015.
“We thought pharmaceutical companies like Merck and Organon were in the business of advancing science, but they’re actually in the business of advancing their bank accounts,” he adds.
Increasingly desperate for relief from his 40-plus PFS symptoms—which range from penile shrinkage to gynecomastia, tooth loss to elevated glucose levels, muscle atrophy to extreme mood swings—Ro three years ago turned to Michael Zitzmann, MD, at the Center for Reproductive Medicine and Andrology in Münster.
“Patient took finasteride for 3 months in 2015… Patient fears that his penis could vanish. Sometimes no feeling of the presence of this organ. No relationship at this time. No wish for paternity,” Dr. Zitzmann—whose 2018 DAZ interview was headlined Targeting neuroactive steroids—wrote in Ro’s medical records.
“My cognitive abilities have diminished, my IQ has declined,” says Roman. “I used to be able to think on my feet and handle complex tasks with ease. But now I struggle with even the simplest of mental challenges.”
 Anyone living in the US who suffers from PFS should report his or her symptoms to the US FDA. Anyone living outside the US who suffers from PFS should report his or her symptoms to the US FDA as well as to his or her local drug-regulatory authority (DRA), as directed on our Report Your Side Effects page.
Anyone living in the US who suffers from PFS should report his or her symptoms to the US FDA. Anyone living outside the US who suffers from PFS should report his or her symptoms to the US FDA as well as to his or her local drug-regulatory authority (DRA), as directed on our Report Your Side Effects page.
Finally, if you or a loved one are suffering from PFS, and feeling depressed or unstable, please don’t hesitate to contact the PFS Foundation as soon as possible via our Patient Support hotline: social@pfsfoundation.org.
Thank you.


