June 27, 2022
Dear Friends:
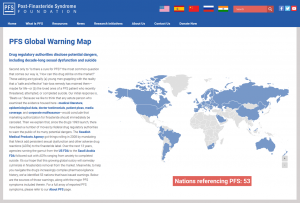 The PFS Foundation has launched an interactive map plotting all the nations known to have issued warnings of finasteride’s potential to cause persistent adverse reactions, aka post-finasteride syndrome (PFS).
The PFS Foundation has launched an interactive map plotting all the nations known to have issued warnings of finasteride’s potential to cause persistent adverse reactions, aka post-finasteride syndrome (PFS).
Additionally, English translations are provided for all non-English texts.
Taken as a whole, the map offers a broader consensus of PFS symptoms than any single DRA. Similarly, it provides insight into how the most vigilant DRAs (a) determine causal associations between finasteride and case reports of adverse reactions, (b) alert health professionals when new clinical research and epidemiological data emerge, and (c) craft regulatory policy based on the potential dangers of finasteride therapy. For instance:
- Canada: In 2018, Health Canada mandated that muscle-related disorders be added to local Propecia (finasteride 1 mg) and Proscar (finasteride 5 mg) product literature. A year later, the agency “concluded that there may be a link between Proscar and Propecia…and the risk of suicidal ideation.”
- China: In 2019, two months after Canada issued its suicidal-ideation warning, the National Medical Products Administration issued a Drug Safety Warning, which noted: “Between 2012 and 2016, the reported rate of finasteride-related suicide/self-harm incidents in Canada increased 2.5-fold… Currently, the US Food and Drug Administration (FDA) has not required changes to the labeling of Proscar or Propecia.”
- Belgium: In 2017, the Federal Agency for Medicines and Health Products (FAMHP) published a flash-news report noting: “New data, analyzed by the European Medicines Agency (EMA), has concluded that the risks of mood disorders, suicidal ideation and depression are possible while taking finasteride at 1 mg/day… It is important that patients are informed of these risks, since no leaflet accompanies the custom prescriptions [i.e., those prepared by pharmacists per doctors’ specifications] of finasteride at 1mg.
- Brazil: In 2019, finasteride manufacturer Merck SA, in agreement with the National Health Surveillance Agency (ANVISA), issued a Letter to Health Professionals informing them that “based on individual patient case reports, sexual dysfunction may…persist for more than 10 years after discontinuation of treatment.” Seventeen years earlier, after assessing the use of finasteride in cosmetics, ANVISA published a technical opinion that noted: “[F]inasteride, when applied topically, has systemic absorption, thus leading to the same side effects when used orally. [The Technical Advisory Committee on Cosmetics] recommends: Prohibit the use of finasteride in cosmetic products.”
- Australia: In 2019, the Therapeutic Goods Administration (TGA) published an interim decision in relation to finasteride, noting: “[T]here is the possibility that finasteride use may result in Post Finasteride Syndrome… [D]own scheduling finasteride to Schedule 3 would set a dangerous precedent for Category X medications, and the potential risks from broader access under Schedule 3 significantly outweigh the benefits.”
- France: In 2019, the Agency for the Safety of Medicine and Health Products (ANSM) “published a fact sheet for men who are currently taking finasteride to treat hair loss, or for whom the prescription is being considered, [which was] developed in conjunction with concerned patient associations and health professionals [and] aims to reinforce patient information on the risk of certain adverse effects, such as psychiatric disorders and/or sexual dysfunction.”
- New Zealand: In 2016, the Medicines and Medical Devices Safety Authority (Medsafe) published a Prescriber Update noting that “Post-Finasteride Syndrome…was recently added to the US National Institutes of Health (NIH) list of genetic and rare diseases.” It also published the full list of reported PFS symptoms from the PFS Foundation’s What Is PFS? page, ranging from anxiety to tinnitus.
- Saudi Arabia: In 2021, the Saudi Food & Drug Authority (SFDA) published a Safety Signal that concluded: “The weighted cumulative evidence identified from the reported cases, literature and data mining are sufficient to support a causal association between Finasteride and the risk of Diabetes Mellitus.” In 2018, Merck Saudi Arabia, in agreement with the SFDA, distributed a Direct Healthcare Professional Communication noting that: “Patients should be aware of the risk of experiencing…sexual dysfunction…during finasteride therapy. Patients should also be informed that case reports have been received of sexual dysfunction…that persisted after discontinuation of therapy… Healthcare professionals should carefully monitor patients during treatment with finasteride for psychiatric symptoms (including anxiety, depression and suicidal ideation).”
- United States: In 2000, in response to Merck’s Citizen Petition to approve finasteride for pediatric use, the Food and Drug Administration (FDA) wrote: “FDA is concerned that use of finasteride by pediatric patients may pose long-term safety risks regarding growth, development and sexual function. Any proposed use in the pediatric population would, of course, need to account for these safety concerns. Accordingly, your petition is denied.”
 Going forward, we plan to update the map (which is housed in the Resources menu on our navigation bar) with new DRA warnings, guidelines, and/or actions about finasteride as soon as they become available.
Going forward, we plan to update the map (which is housed in the Resources menu on our navigation bar) with new DRA warnings, guidelines, and/or actions about finasteride as soon as they become available.
To assist in this effort, we invite interested parties living in countries not currently represented on the map to reach out to their local DRAs and request electronic copies of the most recent finasteride product literature and/or news. All such content should be emailed to Philip Roberts.
Anyone living in the US who suffers from PFS should report his symptoms to the US FDA. Anyone living outside the US who suffers from PFS should report his symptoms to the US FDA as well as to his or her local DRA, as directed on our Report Your Side Effects page.
Finally, if you or a loved one are suffering from PFS, and feeling depressed or unstable, please don’t hesitate to contact the PFS Foundation as soon as possible via our Patient Support hotline: social@pfsfoundation.org
Thank you.
Related News
