Probe launched after tête-à-tête with French counterpart ANSM, whose ‘red box’ warning will be slapped on all finasteride 1 mg packaging next month
March 5, 2023
Dear Friends:
The British are coming—hopefully with a PFS-awareness program on par with that of the French.
According to a February 16 memo from Amelia White, MD, Medical Accessor for the UK’s Medicines and Healthcare products Regulatory Agency (MHRA), the agency is conducting a safety review of finasteride 1 mg.
“This topic was discussed at our recent signal meeting and has led to several action points which we are currently undertaking,” Dr. White told PFS patients who have complained to the agency about finasteride. Those points, she continued, include:
• Assessment of the available scientific literature
• Review of the documentation on safety and risk required to be produced by marketing authorisation holders under the Human Medicines Regulation 2012
• Consideration and review of the adverse drug reaction reports
• Review of assessments and actions undertaken by other regulatory authorities
• Consideration of concerns raised to our attention via other sources such as healthcare professionals, individuals, patient representative groups, other stakeholders
• Engagement with relevant clinical and scientific experts, patients and patient representative groups
Following French pharmacovigilance?
Dr. White also informed patients that the MHRA has been in contact with its French counterpart, L’Agence nationale de sécurité du médicament et des produits de santé (ANSM) about finasteride-safety issues. But “we will need to complete our review before we can determine whether proportionate regulatory action is required here in the UK,” she noted.
In November (as we reported here), ANSM unveiled plans (English) to add a so-called “red-box” warning—that includes a QR code—on all finasteride 1 mg products by April 28.
Intended to “reinforce information on adverse effects” of Propecia and generics, the warning reads:
This medication can cause side effects, including psychiatric and/or sexual disorders. To find out more about these effects and report them, consult the leaflet, and scan this QR code.
Arguably the most thorough and vigilant PFS-prevention campaign ever produced by a drug-regulatory authority (DRA), ANSM’s content was developed in collaboration with French patient-advocacy group Aide for Victims of Finasteride (AVFIN), and is housed on the ANSM website in six parts.
Part 6 is an interactive video that “provides a step-by-step guide for anyone wishing to report an adverse reaction potentially linked to [finasteride]” to the French Ministry of Health’s portal for adverse health events. Notably, the array of possible adverse reactions presented on page 11 of the video is divided into 44 disorders, ranging from insomnia to tinnitus to memory loss to pudendal neuralgia to attempted suicide.
The insider
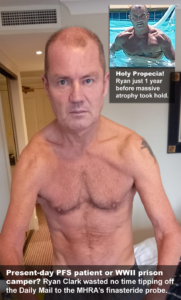 In what appears to be an AVFIN-like movement taking root in the UK, PFS patients there have begun rallying around fellow sufferer Ryan Clark, 56.
In what appears to be an AVFIN-like movement taking root in the UK, PFS patients there have begun rallying around fellow sufferer Ryan Clark, 56.
Ryan, who spent most of his career employed by Her Majesty’s Government, first contacted the MHRA in June 2021 to let them know how finasteride had decimated his health, and warn that countless other British citizens taking the drug are at risk of a similar fate.
A month later (as we reported here), MHRA Scientific Assessor Alison Banner Simpson responded:
In line with other regulators worldwide the MHRA considers that based on the currently available data on quality, safety, and efficacy the balance of benefits and risks of finasteride is positive.
Long story short, Ryan continued to petition the MHRA for tighter restrictions on finasteride, and more vigorous warnings on its packaging, but was similarly rebuffed over the next 18 months.
Only after he complained that Sons, an online pharmacy touting itself as “Next generation men’s health and well-being,” completely glossed over the drug’s potential to cause psychological and cognitive damage did the MHRA take action. That, in the form of a decision published December 2021, which read, in part:
MHRA contacted Careforsons Limited regarding the concerns and asked them to review their website to ensure readers are given a balanced overview of the potential risks and benefits of using this medicine and to avoid absolute statements of safety.
Little left to lose
Once buff enough to grace the cover of a men’s fitness magazine, Ryan’s body has been gradually wasting away from a dizzying array of health woes he attributes to PFS. Chief among them: adrenal dysfunction, autonomic dysfunction, androgen deprivation, benign tumors, chronic insomnia, cognitive dysfunction, dermatological disorders, gastrointestinal disorders, generalized anxiety disorder, global soft tissue atrophy, muscle atrophy, neuropathy, suicidal ideation, and sexual dysfunction.
So when Dr. White contacted him last month about the MHRA’s finasteride probe—and follow-up with an in-depth interview about the growing incidence of PFS in her nation—Ryan didn’t hesitate to share that “good” news with the Daily Mail.
 In turn, The Mail yesterday ran a 1,100-word exclusive by Senior Health Reporter Ethan Ennals headlined, Watchdog Launches Investigation into Hair Loss Pill as Men Report Huge Rise in Side Effects Including Depression, Low Libido and Erectile Dysfunction.
In turn, The Mail yesterday ran a 1,100-word exclusive by Senior Health Reporter Ethan Ennals headlined, Watchdog Launches Investigation into Hair Loss Pill as Men Report Huge Rise in Side Effects Including Depression, Low Libido and Erectile Dysfunction.
Simultaneously, the paper debuted an episode of its companion podcast, Medical Minefield, co-hosted by Ethan and titled Just how safe is the hair-loss drug taken by millions of men?
In his story, Ethan writes, “Government figures…show that the drug finasteride has been linked with at least 70 reports of patients suffering suicidal thoughts as a suspected side effect.”
And on the podcast he notes, “In the last three years, the number of people reporting adverse side effects to the medical authorities in the UK has tripled.”
Ethan also talks up the $2 billion telemed firm Hims & Hers Health (NYSE: HIMS):
Hims, which is one of the major companies that sells [finasteride] in the UK, doubled their profits in the last year. Very friendly, Instagram-friendly, TikTok friendly… A lot of their ads air during football matches. There’s this quite notorious one that says, “Stop the despair, despair, despair of hair loss.” They don’t mention it, but you go to their website and you can order the drug.
And that’s basically in line with when these online firms started selling finasteride on the web, around the same time as COVID, when everything went online.
Ethan’s comments come less than a week after we reported on the case of Sumair Ahluwalia, a 21-year-old college student from Chicago who developed PFS shortly after using a non-FDA-approved topical-finasteride product prescribed by a Hims nurse practitioner (Topical Finasteride Could Precipitate PFS, Top German Rx Journal Warns).
Pyrrhic victory
Thanks to Ryan, The Mail’s more than 800,000 readers were this weekend informed that the British government is now devoting time and resources to developing a clearer picture of the PFS epidemic.
“People need to be properly informed about the risks attached to finasteride before taking it,” he tells the paper, which dubs him a “patient advocate.”
But for Ryan, the accomplishment is bittersweet.
“These side effects have been devastating and made me progressively worse over the years,” he says.
If you’d like to learn more about navigating pharmacovigilance channels and/or help Ryan organize PFS awareness efforts in the UK, feel free to contact him at clarkpod1@hotmail.co.uk.
 Anyone living in the US who suffers from PFS should report his or her symptoms to the US FDA. Anyone living outside the US who suffers from PFS should report his or her symptoms to the US FDA as well as to his or her local drug-regulatory authority (DRA), as directed on our Report Your Side Effects page.
Anyone living in the US who suffers from PFS should report his or her symptoms to the US FDA. Anyone living outside the US who suffers from PFS should report his or her symptoms to the US FDA as well as to his or her local drug-regulatory authority (DRA), as directed on our Report Your Side Effects page.
Finally, if you or a loved one are suffering from PFS, and feeling depressed or unstable, please don’t hesitate to contact the PFS Foundation as soon as possible via our Patient Support hotline: social@pfsfoundation.org.
Thank you.
Related news

