Aug. 4, 2022
Dear Friends:
This month marks a full decade that we’ve been in the business of facilitating PFS research, generating awareness of the condition, providing support to patients suffering from the condition, and lobbying for stricter regulation so that fewer unsuspecting men develop the condition.
 Now, if at any time between August 2012 and August 2021, you’d asked us to sum up the PFS situation in one word, we might have said distressing. Or tragic. Or horrific.
Now, if at any time between August 2012 and August 2021, you’d asked us to sum up the PFS situation in one word, we might have said distressing. Or tragic. Or horrific.
But absurd? Never.
That all changed in September 2021, however, when we learned that Merck & Co. had three months earlier spun off its Organon & Co. subsidiary as an independent, publicly traded company. And that among Organon’s “broad portfolio of important medicines” were Propecia and Proscar.
So we logged onto the Organon website to see what kind of company had so kindly taken the original finasteride products off Merck’s hands. What we found was a company whose tagline is “Here for her health.”
Reading further, we learned “Why we’re here,” which is:
We believe in a better and healthier every day for every woman. And we understand that women are foundational to a healthier world. As a new company, we will begin by listening to women’s healthcare needs, big and small, enabling us to develop treatments tailored to them—because we know there is so much more we can do for women and their health.
Okay. But second only to Viagra and Cialis, finasteride is perhaps the most male-oriented medicine on earth. So what on earth are Propecia and Proscar doing on Organon’s product page, tucked neatly beneath Pregnyl—which, incidentally, is a hormone that supports the normal development of an egg in a woman’s ovary?
That’s like Home Depot adding a section, to all 2,317 stores worldwide, called “Intimate Apparel.” And hung neatly in that section, smack dab between Lumber and HVAC Services, are lace thongs and push-up bras.
Absurd indeed.
On that note, we’d like to share some highlights from the past 12 months.
REGULATORY ACTIVITY
If American authorities are increasingly cagey about the many dangers finasteride poses to human health, European authorities are making up for it by being increasingly attentive to this mini epidemic.
France
Last month, the National Agency for the Safety of Medicines and Health Products (ANSM) released a dossier of educational materials on the growing number of adverse drug reactions (ADRs) to finasteride 1 mg, as experienced by PFS patients. Easily the most thorough and vigilant PFS-prevention campaign ever produced by a drug-regulatory authority (DRA), the content was developed in collaboration with French patient-advocacy group Aide for Victims of Finasteride (AVFIN), and is housed on ANSM’s website in six parts:
 1. Finasteride 1 mg for the treatment of early-stage hair loss (English): “Considering the impact that some of the adverse reactions associated with finasteride can have on the patient’s quality of life, it is essential to be aware of them before starting treatment. By the same token, it is important that a rigorous and regular medical follow-up is conducted during treatment.”
1. Finasteride 1 mg for the treatment of early-stage hair loss (English): “Considering the impact that some of the adverse reactions associated with finasteride can have on the patient’s quality of life, it is essential to be aware of them before starting treatment. By the same token, it is important that a rigorous and regular medical follow-up is conducted during treatment.”
2. Finasteride 1 mg and hair loss (English): “[A]lthough the desired action of finasteride is the reduction of the level of DHT in hair follicles, other organs can be impacted. If drugs based on finasteride 1 mg manage…to combat androgenetic alopecia, the hormonal action of finasteride could be the cause of sometimes severe adverse effects.”
3. Risks of taking finasteride 1 mg (English): “Patients have reported sexual disorders when using finasteride 1 mg… Patients have also reported mental disorders, including anxiety, depression, [and] suicidal thoughts that could lead to suicide… All of these disorders can have an impact on social and professional life. Sexual and psychological disorders may persist after stopping treatment for an indefinite period.”
4. Information for patients treated with finasteride 1 mg (English): “It is important to remain vigilant for the appearance of worrying/unusual signs, even if they seem trivial to you… It is also recommended that you inform your relatives that you are taking finasteride…and ask them to alert you if they observe any changes in your behavior.”
 5. Information for healthcare professionals about finasteride 1 mg (English): “Remember that finasteride…can cause mental disorders and sexual disorders… [I]t is advisable to schedule a follow-up consultation within three months after initiation of treatment…then regularly (for example, every six months) during treatment.”
5. Information for healthcare professionals about finasteride 1 mg (English): “Remember that finasteride…can cause mental disorders and sexual disorders… [I]t is advisable to schedule a follow-up consultation within three months after initiation of treatment…then regularly (for example, every six months) during treatment.”
6. How to report adverse reactions to finasteride: “[T]his interactive video…provides a step-by-step guide for anyone wishing to report an adverse reaction potentially linked to [finasteride]” to the French Ministry of Health’s portal for adverse health events. Notably, the array of possible adverse reactions presented on page 11 of the video (above) is divided into 44 disorders, ranging from insomnia to tinnitus to memory loss to pudendal neuralgia.
United Kingdom
The Medicines and Healthcare products Regulatory Agency (MHRA), meanwhile, is also giving PFS patients a louder voice in the pharmacovigilenace process. In December, based on evidence submitted by Ryan Clark, the agency published a decision titled September 2021 Promotion of finasteride by Careforsons Limited t/a Sons. It read, in part:
 A member of the public contacted MHRA to raise concerns about the information provided for finasteride on the Sons website. The complainant did not consider that the mental-health side-effects and emotional wellbeing were sufficiently or appropriately discussed on the webpages where this prescription-only medicine was highlighted. MHRA contacted Careforsons Limited regarding the concerns and asked them to review their website to ensure readers are given a balanced overview of the potential risks and benefits of using this medicine and to avoid absolute statements of safety.
A member of the public contacted MHRA to raise concerns about the information provided for finasteride on the Sons website. The complainant did not consider that the mental-health side-effects and emotional wellbeing were sufficiently or appropriately discussed on the webpages where this prescription-only medicine was highlighted. MHRA contacted Careforsons Limited regarding the concerns and asked them to review their website to ensure readers are given a balanced overview of the potential risks and benefits of using this medicine and to avoid absolute statements of safety.
Among the offending statements found on Sons’ Finasteride FAQs page are:
● “Finasteride is safe.”
● “Finasteride doesn’t stay in your system for very long once you stop taking it… In fact, on stopping the medication, it is usually completely excreted after seven days.”
● “For those people that do experience mild side effects, improvement usually follows after a short while.”
“I urge anyone who believes that nothing can be done about the PFS epidemic to begin educating him- or herself immediately on the mechanisms in place at their own drug agencies for bringing the horrors of this condition to light,” Clark, 54, who hails from the northeast of England—and has endured decades of symptoms running the gamut from tooth loss to testicular cancer—told us. (His full story here.)
DIGITAL GROWTH
Warning Map
Encouraging as it is to see drug-regulatory authorities issue new finasteride warnings, such information is often nearly impossible for those who need it most—patients and health professionals—to locate. Because there are no universal standards for reporting adverse drug reactions. Because not all DRAs make their ADR reports readily available to the public. Because not all DRAs provide translations of pertinent texts into one or more of the four languages (English, Chinese, Hindi, Spanish) whose native speakers exceed 500 million. And because there is no digital portal where all public records of all DRA activity associated with all prescription medications are housed.
And yet, every human being on earth who takes finasteride ingests precisely the same chemical substance.
 So we created the PFS Global Warning Map. Launched in June and housed in the Resources menu of our website, this interactive map plots all the nations known to have issued warnings of finasteride’s potential to cause persistent adverse reactions. Fifty-four of them, from Argentina to the US, are currently represented. Each links to a table entry that in turn links to at least one original document from the respective nation’s DRA, with English translations provided for all non-English texts.
So we created the PFS Global Warning Map. Launched in June and housed in the Resources menu of our website, this interactive map plots all the nations known to have issued warnings of finasteride’s potential to cause persistent adverse reactions. Fifty-four of them, from Argentina to the US, are currently represented. Each links to a table entry that in turn links to at least one original document from the respective nation’s DRA, with English translations provided for all non-English texts.
Taken as a whole, the map offers a broader consensus of PFS symptoms than any single DRA. Similarly, it provides insight into how the most vigilant DRAs (a) determine causal associations between finasteride and case reports of adverse reactions, (b) alert health professionals when new clinical research and epidemiological data emerge, and (c) craft regulatory policy based on the potential dangers of finasteride therapy. For instance:
● Brazil: In 2019, finasteride manufacturer Merck SA, in agreement with the National Health Surveillance Agency (ANVISA), issued a Letter to Health Professionals informing them that “based on individual patient case reports, sexual dysfunction may…persist for more than 10 years after discontinuation of treatment.” In 2002, after assessing finasteride use in cosmetics, ANVISA published a technical opinion noting: “[F]inasteride, when applied topically, has systemic absorption, thus leading to the same side effects when used orally. [The Technical Advisory Committee on Cosmetics] recommends: Prohibit the use of finasteride in cosmetic products.”
● Australia: In 2019, the Therapeutic Goods Administration (TGA) published an interim decision in relation to finasteride noting: “[T]here is the possibility that finasteride use may result in Post Finasteride Syndrome… [D]own scheduling finasteride to Schedule 3 would set a dangerous precedent for Category X medications, and the potential risks from broader access under Schedule 3 significantly outweigh the benefits.”
● New Zealand: In 2016, the Medicines and Medical Devices Safety Authority (Medsafe) published a Prescriber Update noting: “Post-Finasteride Syndrome…was recently added to the US National Institutes of Health (NIH) list of genetic and rare diseases.” It also published the full list of reported PFS symptoms from the PFS Foundation’s What Is PFS? page, ranging from anxiety to tinnitus.
● Saudi Arabia: In 2021, the Saudi Food & Drug Authority (SFDA) published a Safety Signal that concluded: “The weighted cumulative evidence identified from the reported cases, literature and data mining are sufficient to support a causal association between Finasteride and the risk of Diabetes Mellitus.” In 2018, Merck Saudi Arabia, in agreement with the SFDA, distributed a Direct Healthcare Professional Communication noting: “Healthcare Professionals should carefully monitor patients during treatment with finasteride for psychiatric symptoms (including anxiety, depression and suicidal ideation).”
● Canada: In 2018, Health Canada mandated that muscle-related disorders be added to local Propecia (finasteride 1 mg) and Proscar (finasteride 5 mg) product literature. A year later, the agency “concluded that there may be a link between Proscar and Propecia…and the risk of suicidal ideation.”
● China: In 2019, two months after Canada issued its suicidal-ideation warning, the National Medical Products Administration issued a Drug Safety Warning noting: “Between 2012 and 2016, the reported rate of finasteride-related suicide/self-harm incidents in Canada increased 2.5-fold… Currently, the US Food and Drug Administration (FDA) has not required changes to the labeling of Proscar or Propecia.”
The Red Dragon
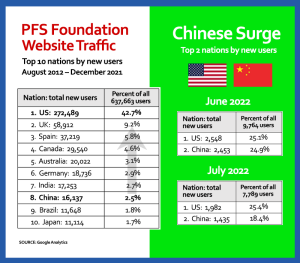 Despite frosty US-Chinese political relations (or maybe because of them), China has been embracing PFS awareness at a record pace. For the month of July 2022, according to Google Analytics, PFSFoundation.org gained 7,789 new users. Of them, 1,982 (25.1%) were from the US, making it our single largest source of new users.
Despite frosty US-Chinese political relations (or maybe because of them), China has been embracing PFS awareness at a record pace. For the month of July 2022, according to Google Analytics, PFSFoundation.org gained 7,789 new users. Of them, 1,982 (25.1%) were from the US, making it our single largest source of new users.
No mystery there. Because in the 10 years since our 2012 launch, the trend has been US No. 1, with China never breaking into the top five. The chart at left (or above on mobile) shows the Red Dragon at year-end 2021, ranking No. 8 with 16,137 new users, versus the US’s 272,489 new users, or 2.5% versus 42.7% of all new users.
Cut to June of this year. China is now neck-and-neck with the US, each generating about 25% of that month’s 9,764 total new users. Cut again, to July, and China has retained an 18% share to the US’s 25%.
The seeds for such dramatic growth were first sown in September 2019, when username Hawk Won posted links to our website on Zhihu, a Chinese question-and-answer platform that boasts 100+ million active monthly users. Two years later, following the announcement of our Baylor College of Medicine gene-expression study, another Zhihu user, Silent Night Watchman—who suffers from PFS—posted a blog titled Reasons for relapse and persistent side effects after using finasteride. That in turn triggered a tidal wave of responses, sending traffic to the Chinese edition of our website up 1,131% month over month and, in less than a week, referring more than 4,000 new Chinese users to us.
RESEARCH
Until now, all the medical literature we’ve referenced in our newsfeed and housed on our website was done so only after it was published in well-established, peer-reviewed journals like JAMA Dermatology, Endocrine Connections, Pharmacotherapy, and the Journal of Sexual Medicine. Going forward, however, when promising findings emerge from researchers with strong track records, we’ll do our best to share it with you in what’s known as poster form.
A poster is a concise summary of research designed to be presented, typically at a professional or academic conference, to fellow experts in the respective field. At such events, the peer-review process often gets under way as the experts ask questions and provide feedback. The researcher later produces a complete study and submits it to a medical journal, which in turn sends it to yet more experts for peer review.
The good news is, when a study is finally published, its findings are highly reliable. But the time-frame between poster and publication can range from several months to several years. And, as we’re painfully aware, PFS patients sometimes abandon all hope during that period.
So by letting you know about select research at the poster stage, we’re hoping to instill hope. On the other hand, please be aware that, in worst-case scenarios, posters never make it to publication because experts deem the science to be weak, flawed, or otherwise unacceptable.
Now let’s talk about two recent posters. And one pill progressing toward FDA approval.
Building on Baylor
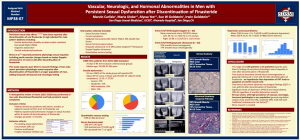 In May, at the American Urological Association Annual Meeting in New Orleans, Irwin Goldstein, MD, director of San Diego Sexual Medicine, made a presentation (at right, or above on mobile) titled Vascular, Neurologic, and Hormonal Abnormalities in Men with Persistent Sexual Dysfunction after Discontinuation of Finasteride.
In May, at the American Urological Association Annual Meeting in New Orleans, Irwin Goldstein, MD, director of San Diego Sexual Medicine, made a presentation (at right, or above on mobile) titled Vascular, Neurologic, and Hormonal Abnormalities in Men with Persistent Sexual Dysfunction after Discontinuation of Finasteride.
“This study expands upon [Mohit] Khera’s research findings of men who report persistent physiologic sexual sequelae after discontinuation of finasteride in a larger population of men, adding grayscale ultrasound and neurologic testing,” he writes.
With 96 patients in the sample group, making it the largest-ever PFS clinical study, Dr. Goldstein’s research:
● Is first to document erectile tissue inhomogeneity on grayscale ultrasound, hypothesizing “that deprivation of DHT leads to [genetically programmed death] of smooth muscle cells.”
● Is first to document abnormal neurological findings.
● Demonstrates “significant levels of depression and sexual distress regardless of [ED] severity.”
● Concludes that ED is “severe, with high prevalence of vascular, neurologic, hormonal pathologies.”
“This is an extremely important study further demonstrating the negative physiologic changes that can occur with finasteride and DHT deprivation within the penile tissue,” Dr. Khera, who was not involved in the research, told us.
“While our prior study demonstrated penile vascular changes in men with PFS, this new study offers a possible mechanism for these vascular changes. In addition, the new study further demonstrates the negative impact finasteride can have on sensory function and hormone levels in men with PFS.”
Adrenaline angle emerges
Also in May, at the 24th European Congress of Endocrinology in Milan, Roberto Cosimo Melcangi, PhD, who heads the Neuroendocrinology Unit of the Department of Pharmacological and Biomolecular Sciences at the University of Milano, made a presentation titled Finasteride Inhibits Epinephrine Synthesis in Humans: Implication for Sexual Dysfunction.
He concluded that:
[F]inasteride affects epinephrine [aka adrenaline] synthesis by blocking PNMT enzymatic activity in humans. This block can have an impact in sexual behavior, as suggested in an animal model treated with finasteride. In addition, the alterations observed in [the mass of erectile tissue forming the bulk of the penis] indicate possible impairment of erectile function. Our results suggest possible mechanisms for the sexual dysfunction observed after finasteride treatment in humans and add a piece of knowledge on the mechanisms controlling sexual function in mammals.
Promising depression therapy
 In addition to facilitating research, the PFS Foundation monitors FDA trials for potentially promising products in clinical development. Sage Therapeutics has allopregnanolone and allopregnanolone analogue products that are currently in trials for various indications, including major depressive disorder (MDD) and insomnia.
In addition to facilitating research, the PFS Foundation monitors FDA trials for potentially promising products in clinical development. Sage Therapeutics has allopregnanolone and allopregnanolone analogue products that are currently in trials for various indications, including major depressive disorder (MDD) and insomnia.
Four years ago, Sage announced an expedited development plan for zuranolone (SAGE-217). Then-CEO Jeff Jonas said at the time that the drug, if approved, “may rewrite the textbook on how the tens of millions of people suffering from MDD are treated, ultimately turning depression into a disorder, not an identity.”
Cut (once again) to May and Sage, now partnered with Biogen, has issued its first zuranolone update in more than two years. The biotech duo:
initiated a rolling submission of a New Drug Application (NDA) to the [FDA] for zuranolone in the treatment of MDD… Data from the completed studies of zuranolone in [our] clinical development programs…as well as data from the completed clinical pharmacology studies, will comprise the full submission package. The rolling submission process allows completed sections of an NDA to be submitted to the FDA for review on an ongoing basis.
“We believe the results from [our clinical development] programs, in which zuranolone demonstrated rapid and sustained effects and a well-tolerated safety profile in clinical trials, support zuranolone as a potential novel treatment option for MDD,” said current CEO Barry Greene.
Sage and Biogen expect to complete submission of the zuranolone NDA by year-end.
PS: PFS finally formalized
 In October, a decade after the first peer-reviewed article on persistent adverse sexual effects in finasteride patients appeared in medical literature, the first-ever diagnostic criteria for PFS followed suit.
In October, a decade after the first peer-reviewed article on persistent adverse sexual effects in finasteride patients appeared in medical literature, the first-ever diagnostic criteria for PFS followed suit.
Titled Diagnostic criteria for enduring sexual dysfunction after treatment with antidepressants, finasteride and isotretinoin, the research by David Healy, MD, a professor of psychiatry at McMaster University, was published in the International Journal of Risk & Safety in Medicine. Dr. Healy led a multidisciplinary team of 36 doctors and researchers from 21 institutions in developing the criteria. Among them are Dr. Khera, Prof. Melcangi, and Michael S. Irwig, MD, of Harvard Medical School.
Healy concludes:
We hope these criteria will lead to increased awareness of the conditions, more accurate diagnoses, and greater research efforts into treatment options for those affected… It is hoped they will ultimately be replaced by diagnostic markers, whether genetic, electrophysiological, endocrine or other.
We’ve housed the full PFS section of the article (which also covers post-SSRI sexual dysfunction, persistent genital arousal disorder following serotonin reuptake inhibitors, and post-retinoid sexual dysfunction) on a new Diagnostic Criteria page in the What Is PFS menu of our website.
(To access a complete list of reported PFS symptoms, please visit our About Post-Finasteride Syndrome page.)
MEDICAL AWARENESS
 We continue to monitor and publish ADR data for finasteride housed in the World Health Organization’s VigiBase database so that health professionals, the public, and members of the media are aware of global trends. Once again, many of the key indicators have risen year over year, with suicide attempts leading the pack at 7.6%, followed by suicidal ideation (7.3%), psychiatric disorders (6.1%), depression (5.4%), and completed suicides (3.4%). The absolute number of 723 new ADR reports during this period translates into two likely cases of PFS reported each day to a DRA.
We continue to monitor and publish ADR data for finasteride housed in the World Health Organization’s VigiBase database so that health professionals, the public, and members of the media are aware of global trends. Once again, many of the key indicators have risen year over year, with suicide attempts leading the pack at 7.6%, followed by suicidal ideation (7.3%), psychiatric disorders (6.1%), depression (5.4%), and completed suicides (3.4%). The absolute number of 723 new ADR reports during this period translates into two likely cases of PFS reported each day to a DRA.
In January, we also began tracking a new ADR, this one known simply as “death,” and categorized under “General disorders and administration site conditions.” That, according to the National Institutes of Health’s National Library of Medicine, is “a class of disorders that encompasses conditions of a general kind that result from a disease, the treatment of disease or administration of treatment at a particular site and are manifested by a characteristic set of symptoms and signs.”
Finasteride is currently linked to 572 such deaths.
Clinicians, pharmacologists, and researchers, increasingly via social media, continue to voice their concerns about finasteride, particularly for young men. Year over year, the number of such professionals aggregated on our Doctors & Researchers Speaking Out page rose 40.5%, from 74 to 104.
 Among the new entrants is Vicente Coutinho, MD, of VPC Medicina Integrada in São Paulo, Brazil, who in January tweeted, “Nowadays some young men prefer to take fina[steride] AND duta[steride] to try to keep their hair. And they know the risks but prefer the hair. It’s insane.”
Among the new entrants is Vicente Coutinho, MD, of VPC Medicina Integrada in São Paulo, Brazil, who in January tweeted, “Nowadays some young men prefer to take fina[steride] AND duta[steride] to try to keep their hair. And they know the risks but prefer the hair. It’s insane.”
Two months later, Tarek Pacha, DO, of Memorial Healthcare Urology in Owosso, MI, tweeted, “Finasteride (Proscar, Avodart, dutasteride) is absolute garbage… Don’t let any man you know take that poison!”
Six months after that, Todd Lee, MD, Associate Professor of Medicine at McGill University in Montreal, tweeted, “While finasteride may reduce incidence of BPH and/or change the incidence or type of prostate cancer one gets, it also seems to increase the risk of self harm and/or suicide in both younger and older men.”
MEDIA AWARENESS
A new documentary examining three young men’s reactions to rapidly thinning locks—shaving, transplanting, and finasteride—featured an interview with Prof. Melcangi. Titled Plötzlich kahl (Suddenly Bald), the 30-minute film by Gustav Hofer (English-subtitled version here) debuted in December on SRF, the largest German-language broadcaster in Switzerland.
 In it, Melcangi says of his numerous rat studies:
In it, Melcangi says of his numerous rat studies:
We can say with certainty that the animals were healthy before finasteride treatment. After treatment, we observed some negative side effects, many of which persisted after quitting the drug. Just like the PFS patients, the rats developed depression, erectile dysfunction, and there are also changes in their intestinal flora.
Importantly, he adds, “The attentiveness of doctors today is certainly much greater. They are more cautious than in the past.”
Suddenly Bald marks the third German-language PFS report on network TV in less than a year. The previous two are: Tricks of the Beauty Industry (WDR, Germany) and The Side Effects of Finasteride Are Underestimated (NDR, Germany).
(For a complete listing of PFS press coverage, please visit our PFS Media Awareness page.)
PATIENT SERVICES
 The number of doctors, psychologists and pharmacologists volunteering to counsel PFS patients as members of our Medical Professionals team rose 4% this year, from 117 to 122. They join us from 25 nations across 17 specialties, and many continue approaching us unsolicited. From Donal O’Shea, MD, in Dublin to Wai Kit Ma, MD, in Hong Kong to Robert Brannigan, MD, in Chicago to Kevin Billups, MD, in Nashville to Mark Nestor, MD, in Miami, we thank them one and all for their efforts in helping PFS patients remain stable and hopeful.
The number of doctors, psychologists and pharmacologists volunteering to counsel PFS patients as members of our Medical Professionals team rose 4% this year, from 117 to 122. They join us from 25 nations across 17 specialties, and many continue approaching us unsolicited. From Donal O’Shea, MD, in Dublin to Wai Kit Ma, MD, in Hong Kong to Robert Brannigan, MD, in Chicago to Kevin Billups, MD, in Nashville to Mark Nestor, MD, in Miami, we thank them one and all for their efforts in helping PFS patients remain stable and hopeful.
Meanwhile, our Patient Support program, designed to connect PFS patients across the globe for moral support and sharing coping strategies, continues to be one of our most popular services. Since its 2016 launch, thousands of afflicted men and their loved ones—who would otherwise have virtually no way of finding one another—are today in regular contact.
PFS PASSING
First thing each day here at the PFS Foundation, we carefully apply our clinical demeanor and hope it remains intact until quitting time. But far too often, with the ring of our iPhone, receipt of an email, or ding of a social-media app, that effort goes up in smoke as we encounter yet another suicidal PFS patient. Even worse is getting word of a patient who succeeded. Worse still is learning that a suicidal patient we thought we’d talked off a ledge just a few months, weeks, or days earlier, marched back up and leapt.
 Such was the case on April 25 when Ryan Clark WhatsApp’d us that Marc James Turner, a 36-year-old staffer with the Parks & Recreation Department in Mississauga, Canada, was gone.
Such was the case on April 25 when Ryan Clark WhatsApp’d us that Marc James Turner, a 36-year-old staffer with the Parks & Recreation Department in Mississauga, Canada, was gone.
We’d first learned of Marc’s existence on Feb. 25, after a friend of his called to say how worried he was about him, and asked if we could help. “Of course! Have him call any time—day or night,” our patient manager replied. “We’ll do whatever we can.”
The next day, Marc did call. He told us that, after taking finasteride for just one month in October 2020, he’d developed severe depression, anxiety, ED, insomnia and “cracking joints.” He also frequently felt suicidal. Yet throughout the conversation, he could not have been more polite, composed, or articulate.
Two days later, Marc asked to participate in our Patient Support program, so we swiftly dispatched a note to all fellow PFS patients and their loved ones in Canada and the US, asking them to reach out to him. We ourselves checked in with Marc several times via email, and on April 4 he replied:
As more clinicians are starting to speak out against the devastating effects of finasteride, have you considered sending out a mass e-mail asking if they would be willing to make a video on YouTube sharing their views on PFS?… I was heavily influenced into thinking the drug was safe by misinformation on YouTube.
Nine days later, Marc took his own life. For those who depress easily, we won’t subject you to the firsthand account of his suffering he published on the social-journalism site Medium (Finasteride Completely Destroyed My Life, June 17, 2021). But anyone else who’s interested can read it here.
Ditto his obituary.
 As we embark on the second decade of our mission, we ask you to continue giving generously to the foundation so we may continue this urgent work.
As we embark on the second decade of our mission, we ask you to continue giving generously to the foundation so we may continue this urgent work.
In the meantime, as directed on our Report Your Side Effects page, anyone living in the US who suffers from PFS should report his or her symptoms to the FDA. Anyone living outside the US who suffers from the condition should also report to the FDA, as well as to his or her local DRA.
Finally, if you or a loved one are suffering from PFS and feeling depressed or unstable, please don’t hesitate to contact us via our Patient Support hotline: social@pfsfoundation.org
Sincerely,
John Santmann, MD
CEO
Philip Recchia
President
Related News
2021 PFS Foundation Annual Address
2020 PFS Foundation Annual Address
2019 PFS Foundation Annual Address
2018 PFS Foundation Annual Address
2017 PFS Foundation Annual Address
2016 PFS Foundation Annual Address
2015 PFS Foundation Annual Address
2014 PFS Foundation Annual Address
2013 PFS Foundation Annual Address
