Aug. 4, 2020
Dear Friends:
Back in March, when COVID-19 first swept America, our patient manager was chatting with a thirtysomething patient who’s been suffering from PFS since 2010. Let’s call him Andrew.
Andrew had recently run into a colleague from the last full-time job Andrew held before developing the condition and being forced to quit. And getting dumped by his fiancée. And giving up his apartment in Brooklyn. And moving back in with his mom. And so on.
The colleague, who knew nothing of Andrew’s PFS, was, like most Americans, in a state of shock over the nightmare that the pandemic, seemingly overnight, had dumped in his lap. His worries went more or less like this:
“One day I’m making decent money managing a restaurant and a week later I’m on the phone five hours a day trying to collect unemployment. Even if I can get it, what happens when my benefits run out?… I just met this girl. I like her a lot and things were going well but she suddenly goes back to Colorado… I spend all day in my apartment alone watching experts say they have no idea when the health crisis will end and no idea how long it’ll take to make a vaccine—if they ever can make one… Some people say no need to wear a mask because the virus is just like a bad cold that soon goes away and young people don’t get it anyway… How is anyone supposed to live like this!”
“Well,” said Andrew, “that’s pretty much how I’ve been living for the past 10 years.”
Now, to mark our eighth anniversary, I’d like to share some highlights from the past one, particularly difficult, year.
RESEARCH
 Loath though we are to focus on it, the darkest side of PFS finally reared its head in medical literature. “Clinicians should be aware that men under the age of 40 who use finasteride for alopecia are at risk for suicide if they develop persistent sexual adverse effects and insomnia,” Michael S. Irwig, MD, attending endocrinologist at Beth Israel Deaconess Medical Center in Boston, wrote in the January issue of Dermatology. The aim of his study, titled Finasteride and Suicide: A Postmarketing Case Series, was to “characterize the clinical histories and symptoms reported by suicide victims who took finasteride to treat hair loss.” In all, Dr. Irwig examined six such cases, based on medical records and autopsy reports provided by family members. Apart from one case who had hyperlipidemia (aka high cholesterol), there was no documentation of concomitant medication use with finasteride, or any baseline medical or psychiatric diagnoses, before starting the drug.
Loath though we are to focus on it, the darkest side of PFS finally reared its head in medical literature. “Clinicians should be aware that men under the age of 40 who use finasteride for alopecia are at risk for suicide if they develop persistent sexual adverse effects and insomnia,” Michael S. Irwig, MD, attending endocrinologist at Beth Israel Deaconess Medical Center in Boston, wrote in the January issue of Dermatology. The aim of his study, titled Finasteride and Suicide: A Postmarketing Case Series, was to “characterize the clinical histories and symptoms reported by suicide victims who took finasteride to treat hair loss.” In all, Dr. Irwig examined six such cases, based on medical records and autopsy reports provided by family members. Apart from one case who had hyperlipidemia (aka high cholesterol), there was no documentation of concomitant medication use with finasteride, or any baseline medical or psychiatric diagnoses, before starting the drug.
A month later, Mohit Khera, MD, Director of the Laboratory for Andrology Research at Baylor College of Medicine in Houston, published a prospective case-control study in Transitional Andrology and Urology titled Penile vascular abnormalities in young men with persistent side effects after finasteride use for the treatment of androgenic alopecia. In it, he reported that “Two subjects (8%) committed suicide during or after the study.” Dr. Khera also noted the presence of vascular abnormalities in the penis of PFS patients (median age 38) who previously discontinued use of 5-alpha reductase inhibitors for hair loss. This marked the first published report of organic pathology in the penis of PFS patients as evaluated by Penile Duplex Doppler Ultrasound, a common clinical test that objectively evaluates sexual function. Dr. Khera’s findings are enormously significant because many physicians dismiss PFS patients’ sexual complaints as psychogenic in nature.
Meanwhile, the European Medicines Agency launched an observational study to assess the extent to which men prescribed 5 mg finasteride for BPH and 1 mg finasteride for male pattern hair loss are at increased risk of recorded suicide and suicide-related outcomes. The study will also assess if any association between finasteride 5 mg exposure and recorded suicidality persists after cessation of therapy. Titled Suicide and suicidality after exposure to finasteride, the study was launched in January and is being conducted by Robert Flynn, MD, in association with the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance.
(For a full directory of PFS-related research, please visit our Medical Literature page.)
REGULATORY ACTIVITY
 French FDA equivalent ANSM continues to set the agenda for PFS awareness across Europe and, hopefully, the world. In December, the agency issued an Information Point and fact sheet (English translation here) for men currently taking finasteride for hair loss, or for those considering taking it for hair loss, designed to supplement the leaflet contained in boxes of finasteride 1 mg. “Developed in conjunction with concerned patient associations and health professionals, this fact sheet aims to reinforce patient information on the risk of certain adverse effects, such as psychiatric disorders and/or sexual dysfunction, associated with finasteride use,” ANSM initially noted in October, adding that “Use of finasteride 1 mg is currently being closely monitored at both the European and national levels.” In a letter issued one month later (English here), ANSM also required MSD France, a subsidiary of finasteride manufacturer Merck & Co., to send the fact sheet to dermatologists, general practitioners and pharmacists with the intention that it be given to patients by doctors when Propecia is first prescribed, and by pharmacists when the drug is dispensed. Topics include:
French FDA equivalent ANSM continues to set the agenda for PFS awareness across Europe and, hopefully, the world. In December, the agency issued an Information Point and fact sheet (English translation here) for men currently taking finasteride for hair loss, or for those considering taking it for hair loss, designed to supplement the leaflet contained in boxes of finasteride 1 mg. “Developed in conjunction with concerned patient associations and health professionals, this fact sheet aims to reinforce patient information on the risk of certain adverse effects, such as psychiatric disorders and/or sexual dysfunction, associated with finasteride use,” ANSM initially noted in October, adding that “Use of finasteride 1 mg is currently being closely monitored at both the European and national levels.” In a letter issued one month later (English here), ANSM also required MSD France, a subsidiary of finasteride manufacturer Merck & Co., to send the fact sheet to dermatologists, general practitioners and pharmacists with the intention that it be given to patients by doctors when Propecia is first prescribed, and by pharmacists when the drug is dispensed. Topics include:
—Sexual Disorders: Patients have reported sexual disorders including erectile dysfunction, ejaculatory dysfunction, testicular pain and decreased libido. These effects may persist after stopping treatment for an indefinite period.
—Psychiatric Disorders: Psychiatric disorders may also occur during finasteride treatment, such as anxiety, depression or even suicidal thoughts. All these disorders can have an impact on your social life.
—How Long Do Adverse Effects Last? The time it takes for adverse events to set in during finasteride treatment may vary, from a few days to a few years after starting treatment. The duration of the adverse effects may also vary widely from patient to patient. Adverse effects may persist after stopping treatment, and in some cases for an indefinite period.
Also in January, ANSM’s Permanent Scientific Committee, which conducts surveillance and pharmacovigilance, issued a report (English here) in which various significant cases of pathologies possibly arising from finasteride therapy are referenced, including meningioma (the most common type of brain tumor), testicular cancer and type II diabetes.
MEDIA AWARENESS
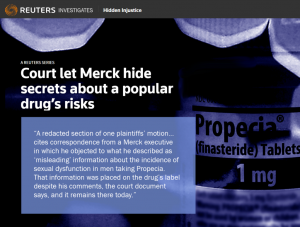 Along with regulatory agencies digging deeper into epidemiological data to better understand the full risks of finasteride use, the media began digging into a question that haunts untold PFS patients across the globe: How was such a drug, which may permanently damage physical, sexual and mental health, ever approved in the first place?
Along with regulatory agencies digging deeper into epidemiological data to better understand the full risks of finasteride use, the media began digging into a question that haunts untold PFS patients across the globe: How was such a drug, which may permanently damage physical, sexual and mental health, ever approved in the first place?
In September, the largest news agency in the Western world published a 3,900-word investigative report headlined Court let Merck hide secrets about a popular drug’s risks. The Reuters story, by Dan Levine, uncovered testimony by former Merck executives in the US Propecia litigation suggesting that the pharmaceutical giant downplayed the drug’s side effects during clinical trials. Though sealed documents including depositions by former Merck executives were partially redacted, Levine was able to read the blacked-out material after copying it from a digital version and pasting it elsewhere. In one such document, Keith Kaufman, MD, head of the Propecia clinical development program, discussed alternate ways of interpreting data from the clinical trials. Referring to language on the Propecia label stating that of those men who took the drug for all five years, “no more than 0.3%” suffered sexual side effects by year five, he called the figure “totally misleading.” Because by year five, “you have weeded out the dropouts with the sexual [adverse experiences].” In another document, Charlotte Merritt, who oversaw regulatory activity for Propecia, was asked if anything prevented Merck from disclosing data they held for over a year at that point about men in the clinical trials “continuing to experience sexual adverse events upon discontinuation.” She responded: “Merck didn’t feel at the time that that was something that needed to be—that needed to be put in the label.”
During his investigation, Levine also turned up the Citizen Petition that the PFS Foundation filed with the US Food and Drug Administration two years earlier, requesting that the agency “immediately require withdrawal of marketing approval for Propecia…because the risk of serious injury from the drug outweighs its limited benefits.” Alternatively, we asked that (a) Boxed Warnings, Precautions and Contraindications to Use be added to the Propecia product label for various serious risks of the drug, including persistent erectile dysfunction, major depressive disorder and male infertility, (b) the FDA make other major product label revisions and (c) the FDA require Merck to develop and implement a Risk Evaluation and Mitigation Strategy Plan. To date, the FDA has not responded to our petition.
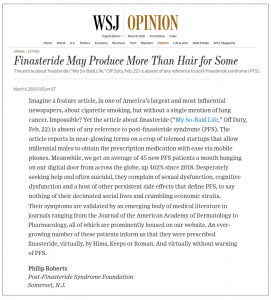 Meanwhile, the foundation helped expose another nightmarish twist in the finasteride saga. In February, The Wall Street Journal ran a 1,280-word story, by Men’s Fashion Editor Jacob Gallagher, headlined Losing Your Hair? Why This Solution Is No Longer Shameful, which read, in part:
Meanwhile, the foundation helped expose another nightmarish twist in the finasteride saga. In February, The Wall Street Journal ran a 1,280-word story, by Men’s Fashion Editor Jacob Gallagher, headlined Losing Your Hair? Why This Solution Is No Longer Shameful, which read, in part:
[In 1991]…effective methods like Rogaine or hair plugs were discussed in hushed tones…That began to change in 2017 with the introduction of a trio of startup brands—Hims, Keeps and Roman—that talked in refreshingly frank, even witty ways about their products’ potential to help men counteract hair loss…Though finasteride is a prescription medication requiring doctor approval, each company offers customers the ease of corresponding with a network of doctors digitally. You fill out a questionnaire, snap some scalp selfies (if required), and if a doctor thinks you need it, they’ll write a prescription which the brand will fill.
Now, you would think that after reading more than 40 clinical studies, meta-analyses and other research demonstrating finasteride’s potential to cause long-term damage to physical, sexual and mental health, the so-called telemeds would shy away from hawking this drug. Yet in the past three years, Hims, Keeps and Roman have collectively raised about $400 million in venture capital, a lot of which goes to marketing campaigns and digital content aimed at painting finasteride as mostly risk-free vis-à-vis persistent side effects. The problem is, more and more of their customers come to us these days complaining of PFS, desperate for help and bewildered at the fact that their respective tele-MD made no mention of the debilitating condition. A few days after we pointed this out to Gallagher, he told us he’d conferred with his bosses, who in turn invited us to submit a Letter to the Editor. One week later, our letter appeared in the WSJ’s Opinion section, headlined Finasteride May Produce More Than Hair for Some. It read, in part:
Imagine a feature article, in one of America’s largest and most influential newspapers, about cigarette smoking, but without a single mention of lung cancer. Impossible? Yet [your] article about finasteride is absent of any reference to post-finasteride syndrome.
 On the social-media front, we were pleased to learn that, thanks to an unknown contributor, Wikipedia competitor Everipedia recently published a PFS Foundation page. Launched in 2015, the site bills itself as The Encyclopedia of Everything and boasts more than three million monthly users.
On the social-media front, we were pleased to learn that, thanks to an unknown contributor, Wikipedia competitor Everipedia recently published a PFS Foundation page. Launched in 2015, the site bills itself as The Encyclopedia of Everything and boasts more than three million monthly users.
Meanwhile on Wikipedia, some other contributor, for the first time ever, recently added a section on long-term sexual dysfunction to the site’s Finasteride page. Among the content referenced there—to support the premise that “[f]inasteride may cause persistent adverse sexual, neurological and physical effects in a subset of men”—are two recent studies: Post-finasteride syndrome: a surmountable challenge for clinicians, by Abdulmaged M. Traish, PhD, Professor of Biochemistry and Urology at Boston University School of Medicine, published in the January 2020 edition of Fertility and Sterility, and Sexual dysfunction in men taking systemic dermatologic medication: A systematic review by George Zakhem, MD, and Julia Goldberg, MD, of the Ronald O. Perelman Department of Dermatology at New York University School of Medicine, published in a March 2019 edition of the Journal of the American Academy of Dermatology (JAAD).
Wikipedia’s expanded Finasteride page also cites the aforementioned Reuters investigation (Court let Merck hide secrets about a popular drug’s risks), noting, among other revelations:
In [one] deposition, Paul Howes, the head of marketing for Propecia, acknowledged that Merck was aware that warnings of sexual side effects, particularly persistent to permanent side effects, would have a devastating impact on sales.
(For a complete listing of PFS press coverage, please visit our Media Awareness page.)
MEDICAL AWARENESS
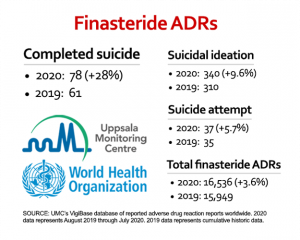 We continue to monitor and publish adverse drug reaction (ADR) data for finasteride housed in the World Health Organization’s VigiBase database so that health care professionals, the general public and members of the media are aware of global trends. Once again, many of the key indicators have risen year over year, with suicides leading the pack at 28%, followed by suicidal ideation at 9.6%, and suicide attempts at 5.7% (see right). The absolute number of 587 new ADRs during this period translates into more than 1.6 likely cases of PFS per day, while the 17 new suicides equate to more than 1.4 a month.
We continue to monitor and publish adverse drug reaction (ADR) data for finasteride housed in the World Health Organization’s VigiBase database so that health care professionals, the general public and members of the media are aware of global trends. Once again, many of the key indicators have risen year over year, with suicides leading the pack at 28%, followed by suicidal ideation at 9.6%, and suicide attempts at 5.7% (see right). The absolute number of 587 new ADRs during this period translates into more than 1.6 likely cases of PFS per day, while the 17 new suicides equate to more than 1.4 a month.
Helping counteract the telemeds’ well-funded efforts to market finasteride as virtually PFS-risk-free, clinicians and pharmacologists worldwide continue to hoist PFS-awareness flags. Year over year, the number of such individuals we’ve aggregated on our Doctors & Researchers Speaking Out page has risen from 41 to 50. Among them is Eduardo García Cruz, MD, of the Serrate & Ribal Urology Institute in Barcelona. Five months ago, in the What is Post-Finasteride Syndrome? section of his practice’s website, Dr. Cruz wrote:
[E]very day I receive more and more emails from young men whose lives have been changed by finasteride. Maybe you think I’m exaggerating. I didn’t believe it either until 10 years ago when I started seeing more men with PFS… I advise you once again to never use [finasteride] to treat hair loss.
Dan Sperling, MD, Medical Director at the Sperling Prostate Center in Delray Beach, FL, also took to his website to sound the PFS alarm. In a post titled BPH? No Fun. Balding? Yuck. But Nobody Warned Me about Finasteride! he wrote:
[T]here is no known treatment for lifelong PFS. Perhaps worst of all, despite that fact that it is the minority of men who are so drastically affected, there is no way to predict who will or won’t suffer the aftermath of taking finasteride. However, PFS is now so prevalent that it has its own foundation, the Post-Finasteride Syndrome Foundation… We believe the warnings about finasteride are justified.
Shelly Gray, meanwhile, a professor at the University of Washington School of Pharmacy, voiced her opinions in one of the world’s leading medical journals. Titled Post-Finasteride Syndrome: Efforts to explain persistent symptoms are undermined by poor long term data on harms, her British Medical Journal editorial reads, in part:
Emerging post-marketing reports of persistent depression and sexual side effects have led to growing concerns about the safety of 5α-reductase inhibitors and prompted product labeling changes in many regulatory jurisdictions. Since 2008, at least 17 countries including the United Kingdom and the United States have warned prescribers of the potential for depression, sexual side effects, or both with finasteride… men should be counselled about the possibility of sexual, physical, and psychological adverse events during treatment and warned that some patients report symptoms after discontinuation.
PATIENT SERVICES
 The number of doctors, psychologists and pharmacologists volunteering to counsel PFS patients as members of our Medical Professionals team rose more than 17% this year, from 85 to a milestone 100. They join us from 26 nations across 16 specialties, and many continue to approach us unsolicited. Case in point: andrologist Rowland Rees, MD, at University Hospital in Southampton, UK. In December he wrote, “I’d be grateful to be considered for your physician finder. I have now seen a number of patients with this problem and it is certainly an area that requires further understanding.” And just last month, urologist Guillaume Altwegg, MD, at Helvetic Care RIVE in Geneva, Switzerland, wrote, “After having known several cases of PFS, I applied myself for a long time to read all the literature on this subject and I have the pleasure of being able to offer personalized care to my patients.” From Kam Mann, MD, in London to Francesco Lombardo, MD, in Rome to Joerg Schueller, MD, in Dubai to Pieter van der Merwe, MD, in Cape Town, we thank them one and all for their ongoing efforts to help PFS patients remain stable and hopeful.
The number of doctors, psychologists and pharmacologists volunteering to counsel PFS patients as members of our Medical Professionals team rose more than 17% this year, from 85 to a milestone 100. They join us from 26 nations across 16 specialties, and many continue to approach us unsolicited. Case in point: andrologist Rowland Rees, MD, at University Hospital in Southampton, UK. In December he wrote, “I’d be grateful to be considered for your physician finder. I have now seen a number of patients with this problem and it is certainly an area that requires further understanding.” And just last month, urologist Guillaume Altwegg, MD, at Helvetic Care RIVE in Geneva, Switzerland, wrote, “After having known several cases of PFS, I applied myself for a long time to read all the literature on this subject and I have the pleasure of being able to offer personalized care to my patients.” From Kam Mann, MD, in London to Francesco Lombardo, MD, in Rome to Joerg Schueller, MD, in Dubai to Pieter van der Merwe, MD, in Cape Town, we thank them one and all for their ongoing efforts to help PFS patients remain stable and hopeful.
Meanwhile, our Patient Support program, designed to connect PFS patients across the globe for moral support and sharing potentially helpful coping strategies, remains one of our most popular services. Since its launch in 2016, thousands of afflicted men and their loved ones—who would otherwise have virtually no way of finding one another—are today in regular contact.
CONTENT MARKETING
 Building on the success of our Spanish-language website, launched in April 2019, we debuted two more international editions. In October came our Mandarin Chinese site, making our content available to more than 1.11 billion speakers of the world’s second most popular language in their native tongue. Then, in May, we debuted our Russian site, allowing 265 million speakers of Europe’s most popular language to read in their native tongue. Couple that with our English reach (1.13 billion) and Spanish reach (543 million) and we’re now able to boast 2.78 billion people worldwide who can readily access our PFS news, medical literature, patient support and other resources.
Building on the success of our Spanish-language website, launched in April 2019, we debuted two more international editions. In October came our Mandarin Chinese site, making our content available to more than 1.11 billion speakers of the world’s second most popular language in their native tongue. Then, in May, we debuted our Russian site, allowing 265 million speakers of Europe’s most popular language to read in their native tongue. Couple that with our English reach (1.13 billion) and Spanish reach (543 million) and we’re now able to boast 2.78 billion people worldwide who can readily access our PFS news, medical literature, patient support and other resources.
Once again, each of our new foreign-language editions was made possible by individuals negatively impacted by PFS. Zhai Cheng Yu of Qingdao, China, whose friend suffers from the condition, translated our entire site into Mandarin pro bono, while Vladimir Zubkov of Kiev, Ukraine, who had a severe reaction to finasteride, did the same on the Russian front.
Thanks largely to our trio of international editions, our website traffic has grown to unprecedented levels. Year over year, unique monthly visitors rose 75%, from 9,548 in July 2019, to an all-time high of 16,505 in July 2020.
As we embark on year eight of our mission to facilitate PFS research, generate PFS awareness and provide support to PFS patients, I ask you to continue giving generously to the foundation so we may continue this urgent work.
 In the meantime, as directed on our Report Your Side Effects page, anyone living in the US who suffers from PFS should report his symptoms to the FDA, and anyone living outside the US who suffers from the condition should report his symptoms to the FDA, as well as to his national drug-regulatory agency.
In the meantime, as directed on our Report Your Side Effects page, anyone living in the US who suffers from PFS should report his symptoms to the FDA, and anyone living outside the US who suffers from the condition should report his symptoms to the FDA, as well as to his national drug-regulatory agency.
Finally, if you or a loved one are suffering from PFS, and feeling depressed or unstable, please don’t hesitate to contact us via our Patient Support hotline: social@pfsfoundation.org
Sincerely,
John Santmann, MD
CEO
Related News
2019 PFS Foundation Annual Address
2018 PFS Foundation Annual Address
2017 PFS Foundation Annual Address
2016 PFS Foundation Annual Address
2015 PFS Foundation Annual Address
2014 PFS Foundation Annual Address
2013 PFS Foundation Annual Address
