Aug. 4, 2023
Dear Friends:
Finasteride was first approved by the US Food and Drug Administration in April 1993. But let’s put that aside for a moment. Let’s instead step back just a dozen years.
In April 2011, I was driving down the Garden State Parkway, on my way from New York City, where I then lived, to the office of Dr. John Santmann in Somerset, NJ, where he then worked. On our agenda was researching post-finasteride syndrome and raising awareness of the condition, efforts that would soon evolve into the PFS Foundation (PFSF).
It was a stunning spring day, cool and clear enough to momentarily nudge the nightmare of PFS from my mind. Suddenly, for no apparent reason, smack dab in the middle of that busy parkway, my engine stopped cold. Silence, save the whoosh of rubber over asphalt.
Unable to accelerate, barely able to steer, and terrified of being rear-ended, I maneuvered best I could onto the grassy roadside. After rolling to a halt, I took a deep breath. Turned the ignition. Engine fired up. Another deep breath. Shifted into forward. Inched back onto the road and made my way safely to Somerset.
 From that day on, my car, a silver Chevy Cobalt I’d bought new in 2006, drove flawlessly. Over the next few years, the mysterious mid-cruise shutdown faded from my memory; a freak incident I was fortunate to survive.
From that day on, my car, a silver Chevy Cobalt I’d bought new in 2006, drove flawlessly. Over the next few years, the mysterious mid-cruise shutdown faded from my memory; a freak incident I was fortunate to survive.
It wasn’t until April 2014, when a postcard from General Motors (NYSE: GM) landed in my mailbox, that I learned there was nothing freak about it. My Cobalt was one of 2.6 million GM vehicles being recalled due to a defective ignition switch that could shut off the engine—and power steering and power brakes and airbags—while driving. Here’s how, according to a congressional investigation, this Motown malfeasance played out:
2001: GM approves its ignition switch for production. 2002: Testing shows the switch to be substandard. Still, it’s installed. 2004: After a Cobalt owner complains that “vehicle can be keyed off with knee while driving,” GM opens an inquiry. 2005: Possible solutions are tossed around, but all are deemed too expensive, too time-consuming, and unable to counteract the defect. GM drops its inquiry. Two months on, another Cobalt owner reports engine shutdown. The inquiry is reopened. An acceptable fix emerges but is later deep-sixed. Two more months on, yet another Cobalt owner suffers shutdown. He crashes. Airbags don’t deploy. He’s killed. Within days, the National Highway Traffic Safety Administration (NHTSA) begins investigating. Four months later, GM issues a service bulletin to dealers advising them to warn owners of possible engine stoppage. 2006: Another Cobalt crashes, this time killing two passengers. 2009: Yet another Cobalt crashes. Two more dead. 2010: GM ceases Cobalt production in North America. 2014: GM recalls at-risk Cobalts. Two months later, after firing 15 senior execs for their roles in the cover-up, newly minted GM CEO Mary Barra testifies before Congress, admitting her company had long fostered a “cost culture” but going forward would shift to a “customer culture that focuses on safety.” A month after that, NHTSA fines GM a record $35 million for failing to recall, despite being aware of 54 crashes and 13 deaths caused by its defective switches.
Horrific and inexcusable as it was, the Cobalt fiasco came and went in one decade. PS: Eight years ago, I traded in my Cobalt for a pre-owned Chevy Tahoe. Six months ago, I bought a new Tahoe.
Now back to finasteride, which, courtesy of Merck & Co. (NYSE: MRK), has been on the market for three decades. According to the World Health Organization, 19,416 finasteride patients to date have reported adverse reactions ranging from 48 different blood and lymphatic system disorders to 75 different vascular disorders. Of those patients, 575 have died, 97 by suicide.
All the while, Merck has never been fined so much as $1 over the finasteride fiasco, not a single Merck staffer has ever been axed, and Kenneth Frazier, who ascended to CEO in 2011, has never testified before congress, let alone admit malfeasance.
What Frazier did do is this: On February 3, 2021, Reuters ran a report headlined Merck Anti-baldness Drug Propecia Has Long Trail of Suicide Reports, which read, in part:
Newly unsealed court documents…show that Merck…and US regulators knew about reports of suicidal behavior in men taking…Propecia when they decided not to warn consumers of those potential risks in a 2011 update of the popular drug’s label… Since the 2011 decision on the warning, the FDA has received more than 700 reports of suicide and suicidal thoughts among people taking Propecia… Those included at least 100 deaths. [I]n the first 14 years the drug was on the market, the agency received 34 such reports, including 10 deaths.
The very next day, suddenly, for no apparent reason, smack dab in the middle of that PR crisis, Frazier announced he was stepping down as CEO.
On that note, I’d like to share some highlights from the past 12, Frazier-free months
REGULATORY ACTIVITY
While it’s too early to say for sure, it looks like pharmacovigilance officers in at least four nations are comparing notes—and realizing that PFS might actually be a mini epidemic.
France
In November, French drug regulatory authority (DRA) ANSM unveiled plans (English) to slap a tech-friendly red-box warning on all finasteride 1 mg products. Intended to “reinforce information on adverse effects” of Propecia and generics, the warning reads:
 This medication can cause side effects, including psychiatric and/or sexual disorders. To find out more about these effects and report them, consult the leaflet, and scan this QR code.
This medication can cause side effects, including psychiatric and/or sexual disorders. To find out more about these effects and report them, consult the leaflet, and scan this QR code.
That QR code links to a six-part dossier of educational materials on the growing number of adverse drug reactions (ADRs) to finasteride 1 mg, as experienced by PFS patients:
1. Finasteride 1 mg for the treatment of early-stage hair loss (English)
2. Finasteride 1 mg and hair loss (English)
3. Risks of taking finasteride 1 mg (English)
4. Information for patients treated with finasteride 1 mg (English)
5. Information for healthcare professionals about finasteride 1 mg (English)
6. How to report adverse reactions to finasteride (video in French only)
In April, however, when the warning was mandated to appear on all new product, Organon & Co. (NYSE: OGN), the Merck spinoff that now owns Propecia (finasteride 1 mg) and Proscar (finasteride 5 mg), refused to play ball for fear of blemishing its corporate brand (tagline: Here for Her Health).
A month later, ANSM issued a news update (English) noting that Organon “has discontinued the marketing of Propecia.” The agency also noted that, although Organon folded its Propecia operations in France, generic versions of finasteride 1 mg are still available at French pharmacies.
Canada
Health Canada (HC) has been actively monitoring finasteride’s potential link to suicidality for more than a decade now. But it wasn’t until 2019 that the DRA detected an epidemiological signal strong enough to take action; namely, updating the finasteride product label to include risk of suicidal ideation.
 In 2022, triggered by Reuters’ 2019 investigative report headlined Court let Merck hide secrets about a popular drug’s risks, HC undertook its fourth finasteride-safety review.
In 2022, triggered by Reuters’ 2019 investigative report headlined Court let Merck hide secrets about a popular drug’s risks, HC undertook its fourth finasteride-safety review.
The probe was completed in January; its findings are delineated, in part, as follows:
• [HC] reviewed 401 cases…of suicide, suicidal ideation and/or self-injury in patients using finasteride from the Canada Vigilance database. Of the 401 cases, 25…met the criteria for further assessment to determine if there was a link between the use of finasteride and suicide, suicidal ideation and self-injury.
• Of the 25 cases, 23…were found to be possibly linked to the use of finasteride.
• [HC] also reviewed 16 publications in the scientific literature. There is a growing body of scientific evidence regarding the association between the use of finasteride and the risks of suicide.
As such, HC:
• is working with the manufacturers to update the product safety information…for finasteride-containing products to strengthen the warning statements on the risks of suicidal ideation and self-injury, and to include information about patient screening for psychiatric risk factors prior to starting treatment, as well as continuous patient monitoring during and after stopping treatment.
• will also inform healthcare professionals about this update through a Health Product InfoWatch communication.
United States
Gone are the days when the US Food and Drug Administration stood as the gold standard of pharmaceutical safety worldwide. Instead, the agency seems to be taking cues from its more vigilant and transparent counterparts in France and Canada.
 Three years after those and other nations first warned of potential links between finasteride use and taking, attempting to take, or thinking about taking one’s own life, Organon finally added “suicidal ideation and behavior” to Propecia’s Prescribing Information (PI, aka product label). Even then, this public disclosure was far from voluntary, and not all that public.
Three years after those and other nations first warned of potential links between finasteride use and taking, attempting to take, or thinking about taking one’s own life, Organon finally added “suicidal ideation and behavior” to Propecia’s Prescribing Information (PI, aka product label). Even then, this public disclosure was far from voluntary, and not all that public.
Organon was directed to add the potentially lethal ADRs as the result of an FDA Citizen Petition filed by the PFSF in 2017, and amended in 2020. In June of 2022, the FDA wrote to inform us:
[B]ased on our analysis of the information contained in part and prompted by your Petition, we are requiring that Organon make labeling changes…to clarify the risks of use of Propecia. We are requiring the addition of suicidal ideation and behavior to the list of nervous system/psychiatric reactions.
Also worth noting is that, although Organon updated its Propecia PI to include suicidality, the drug’s Patient Product Information (PPI, aka Patient Package Insert), wasn’t updated. The PPI housed on Organon’s website as of press time was last revised in October 2021, and makes no reference to suicidality.
Based on precedent, however, it would be easy to argue that Organon should have revised the PPI, a document written in layman’s terms for patients and running just 947 words, along with the PI, a document written in scientific terms for doctors and pharmacists, and running 7,154 words. From 2001 through 2014, according to documents housed on the FDA’s database of approved drugs, Merck did revise each of its Propecia PPIs in tandem with the PI revisions, and those revised PPIs were included at the end of the same PDFs as the PIs. It’s unclear why, in June 2021, Merck and/or the FDA stopped including a corresponding revised PPI in the revised PI file.
Compounding this lack of transparency, the FDA, along with mandating that suicidality be added to the Propecia label, could have mandated that Organon distribute a Dear Health Care Provider Letter (DHCP). But the FDA did not mandate a DHCP as part of its latest Propecia label revision. That means, unless physicians and/or pharmacists happen to see the new suicidality ADRs in the revised PI, and alert their patients accordingly, those patients might never know they are being put in the way of potentially fatal self-harm.
United Kingdom
France and Canada’s steady crack-down on scantily warned access to finasteride has prompted the UK, as well, to act. In February, we learned that the Medicines and Healthcare Products Regulatory Agency (MHRA) is conducting a safety review of finasteride 1 mg.
 “This topic was discussed at our recent signal meeting and has led to several action points,” the doctor conducting the probe wrote in a memo to PFS patients who’ve complained to the agency about finasteride’s many potential dangers. Those points include:
“This topic was discussed at our recent signal meeting and has led to several action points,” the doctor conducting the probe wrote in a memo to PFS patients who’ve complained to the agency about finasteride’s many potential dangers. Those points include:
• Assessment of the available scientific literature
• Review of the documentation on safety and risk required to be produced by marketing authorisation holders under the Human Medicines Regulation 2012
• Consideration and review of the adverse drug reaction reports
• Review of assessments and actions undertaken by other regulatory authorities
• Consideration of concerns raised to our attention via other sources such as healthcare professionals, individuals, patient representative groups, other stakeholders
The memo also informed patients that the MHRA has been in contact with ANSM about finasteride safety. But “we will need to complete our review before we can determine whether proportionate regulatory action is required here in the UK,” it noted.
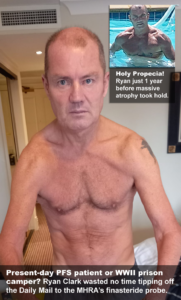 Thanks to one British PFS patient in particular, Ryan Clark, 57, that memo soon became the focus of an exclusive report in the world’s most popular online newspaper.
Thanks to one British PFS patient in particular, Ryan Clark, 57, that memo soon became the focus of an exclusive report in the world’s most popular online newspaper.
On March 4, The Daily Mail ran a story headlined, Watchdog Launches Investigation into Hair Loss Pill as Men Report Huge Rise in Side Effects Including Depression, Low Libido and Erectile Dysfunction. In it, reporter Ethan Ennals noted, “Government figures…show that the drug finasteride has been linked with at least 70 reports of patients suffering suicidal thoughts as a suspected side effect.” And in a companion podcast he added that, since 2020, “The number of people reporting adverse side effects to the medical authorities in the UK has tripled.”
Ryan, meanwhile, who spent most of his career employed by Her Majesty’s Government, first contacted the MHRA in 2021 to let them know how finasteride had decimated his health, and warn that countless other UK citizens taking the drug are at risk of a similar fate. Once buff enough to grace the cover of a men’s fitness magazine, his body is gradually wasting away from a dizzying array of health woes running the gamut from androgen deprivation to gastrointestinal disorders to muscle atrophy to suicidal ideation.
Though his pleas for tighter restrictions on the drug were rebuffed over the next 18 months, he hit pay dirt on February 17 when the MHRA contacted him about its finasteride investigation. To help spread the news, Ryan immediately rang up The Daily Mail.
The MHRA’s findings on finasteride risks will be presented at its August 16 Expert Advisory Group meeting, and an appropriate action plan will be developed soon after.
(For information on how other nations have been monitoring finasteride adverse events, disclosing risks to the public, and regulating the medication, see our PFS Global Warning Map.)
GLOBAL DIGITAL GROWTH
For a medical condition that Organon says doesn’t exist, PFS sure is a hot topic these days, from Albania to Venezuela—at least judging by traffic to our website.
 In May, according to Google Analytics, pfsfoundation.org surpassed 750,000 unique users and two million page views since our 2012 launch. Much of that recent growth has been driven by our four foreign-language editions: Spanish (launched 2019), Chinese (2019), Russian (2020) and Hindi (2021). Of the 85,508 new users to our site from August 1, 2022 to July 31, 2023, 59% came in via our English-language edition, while 41% came in via the overseas editions, up 12% year over year.
In May, according to Google Analytics, pfsfoundation.org surpassed 750,000 unique users and two million page views since our 2012 launch. Much of that recent growth has been driven by our four foreign-language editions: Spanish (launched 2019), Chinese (2019), Russian (2020) and Hindi (2021). Of the 85,508 new users to our site from August 1, 2022 to July 31, 2023, 59% came in via our English-language edition, while 41% came in via the overseas editions, up 12% year over year.
Meanwhile, PFS patients from nations in which we previously knew none continue showing up chez nous. In April, Micha from Kenya wrote us:
I developed full-blown symptoms of PFS in January, after taking finasteride for 15 months. My testosterone dropped to 164 and my testicles have shrunk. I would like to join your organisation to help with the fight against this poison.
Two months later, Hussain from Bahrain wrote:
I am PFS sufferer who took finasteride in 2020 to combat hair loss. To date, I live with almost all the classic symptoms mentioned on your website, both sexual and mental. I have seen 10 doctors (urologists, endocrinologist, sexologist, psychiatrist, GP), but no one seems to offer an actionable solution, and most were not aware of the condition. Can you please connect me to a knowledgeable doctor who has treated patients? At this point of desperation, I’m willing to travel for treatment.
And just last month, Shabanah from Trinidad and Tobago wrote:
My boyfriend (38) was taking Propecia for hair loss for approximately 1 year and started to experience symptoms such as brain fog, erectile dysfunction, low libido, emotional issues. He stopped the medication over 1 year now and the symptoms continue. We were planning to get married and start a family. This has now impacted our life together.
(For those interested following our digital growth, we’ve added a section called “Total website users worldwide” to our PFS by the Numbers page.)
RESEARCH
We’re pleased to report that researchers continue examining PFS at both the micro and macro levels. Let’s begin with two of the latter investigations.
Analyze this
In September, Kian Asanad, MD, Chief Resident Physician in Urologic Surgery at University of Southern California’s Keck School of Medicine (KSM), published a study in the International Journal of Impotence Research titled, Global online interest in finasteride sexual side effects. The research determined that PFS is “clearly a problem that is important to the general public and warrants intervention.”
 Dr. Asanad and his team of three fellow MDs began with the premise that:
Dr. Asanad and his team of three fellow MDs began with the premise that:
72% of Americans look online for health information, and 77% of these used a general (non-medical) search engine such as Google… Qualitative data have shown that patients use web-based health information for everything from direct decision making to guiding discussions with their care team. Understanding these patterns may facilitate a greater understanding and transparency to the patient/physician relationship.
Then, in order to identify global trends vis-à-vis finasteride and its side effects, they plugged the terms “finasteride,” “finasteride side effects,” “post-finasteride syndrome,” “Propecia,” and “Propecia side effects” into Google Trends. Then they compared the total annual number of searches for each term, as conducted in the US, UK and Australia, from 2004 through 2020.
“We observed a significant interest in ‘post-finasteride syndrome.’ There was a steep growth trend, specifically between 2009 and 2012, with an average [annual percent change of] +151.7,” wrote Asanad, noting that 2012 is when the FDA mandated persistent sexual dysfunction be added to the Propecia label.
That “significant interest” becomes even more pronounced when search volumes for the generic name of the prescription hair-loss medication (finasteride) alone, and its brand name (Propecia) alone, are compared to those names within the context of terms that are defined, in part, as having a negative impact on human health. Following are average annual percent changes for searches conducted in the US between 2004 and 2020:
• “Propecia”: –9.8
• “finasteride”: +9.2
• “finasteride side effects”: +20.7
• “post-finasteride syndrome”: +29.2
Out of control
In February, Mayer Brezis, MD, Professor of Medicine Emeritus at the Hebrew University of Jerusalem, published research, based in part on the FDA’s Adverse Event Reporting System (FAERS), demonstrating that finasteride is 23 times as likely to precipitate depression and anxiety as two control medications: Inderal, a beta-blocker linked to depression, and Spironolactone, a potassium-sparing diuretic used to treat alopecia. By the same measures, finasteride use is nine times as likely to bring about insomnia, fatigue, suicidality, and suicide.
 In the paper, titled Neuropsychiatric Reactions to Finasteride: Nocebo or True Effect? and published in the Journal of Basic and Clinical Pharmacy, Dr. Brezis wrote:
In the paper, titled Neuropsychiatric Reactions to Finasteride: Nocebo or True Effect? and published in the Journal of Basic and Clinical Pharmacy, Dr. Brezis wrote:
Reports of adverse reactions increasingly suggest that finasteride may cause depression, anxiety, suicidality and sexual dysfunction, even after discontinuation of the drug. On the other hand, some publications have claimed that this could represent simulated reporting of a nocebo effect. In the present paper, we…demonstrate remarkably disproportionate safety signals for finasteride. Furthermore, the rise in neuropsychiatric reactions to finasteride over the last decade concurs with a striking increase in suicides reported to the FDA in relationship to this medication.
Via FAERS, Dr. Brezis accessed suicidality data for finasteride from 1993 through 2021, which include reports of suicide attempts, suicidal behavior, suspected suicide and completed suicide, the outcomes of which where: died, disabled, hospitalized, life threatening, or other.
The absolute number of such reports peaked in 2020, at 95, falling just 4% in 2021, to 91. From 2019 to 2020, however, the number of such incidents rose 375%. Even more striking is the decade-over-decade data. In 2010, FAERS contained just two suicidality reports for finasteride, which translates into a rise of 4,650% by 2020. And in 2001, FAERS contained just one suicidality report for finasteride, translating into a rise of 9,000% by 2021.
“Since suicide has not been associated with a nocebo effect,” Dr. Brezis noted, “it seems likely that we are facing real and serious adverse effects on mood from finasteride.”
Proof of PFS recovery
On the clinical front, Roberto Cosimo Melcangi, PhD, Head of the Neuroendocrinology Unit in the Department of Pharmacological and Biomolecular Sciences at the University of Milano (UniMi), continues to make headway toward sufficiently mapping PFS in an animal model so to begin considering potential therapies in humans.
His latest study, published in October in Biomolecules and titled Gut Inflammation Induced by Finasteride Withdrawal: Therapeutic Effect of Allopregnanolone in Adult Male Rats, demonstrated that the neurosteroid allopregnanolone (ALLO) proved effective in counteracting some of the finasteride-induced alterations in gut microbiota. Prof. Melcangi wrote:
 [P]revious observations in PFS patients and an experimental model showed alterations in gut microbiota populations, suggesting an inflammatory environment. To confirm this hypothesis, we…explored the effect of chronic treatment with finasteride…and its withdrawal…on the levels of steroids, neurotransmitters, pro-inflammatory cytokines and gut permeability markers in the colon of adult male rat. The obtained data demonstrate that the levels of [ALLO] decreased after finasteride treatment and after its withdrawal.
[P]revious observations in PFS patients and an experimental model showed alterations in gut microbiota populations, suggesting an inflammatory environment. To confirm this hypothesis, we…explored the effect of chronic treatment with finasteride…and its withdrawal…on the levels of steroids, neurotransmitters, pro-inflammatory cytokines and gut permeability markers in the colon of adult male rat. The obtained data demonstrate that the levels of [ALLO] decreased after finasteride treatment and after its withdrawal.
Following the drug suspension, the decrease in ALLO levels correlates with an increase in IL-1 and TNF-, serotonin and a decrease in dopamine. Importantly, ALLO treatment is able to counteract some of these alterations.
[B]ecause (i) sexual dysfunction may be related to alterations in gut microbiota and (ii) the existence of the well-described gut-brain axis, observations here obtained may provide an important background to explore [the] protective effect of ALLO on psychiatric and andrological dysfunctions, laying the groundwork for possible therapy in PFS patients.
More research on deck
Somewhat amazing, but not really surprising, Team Melcangi has simultaneously been working on three separate studies. The first one examines the effects of finasteride on the corpus cavernosum (better known, in males, as the penis). Having made it through one round of peer review at a journal, it was recently revised and resubmitted for publication.
The second study, for a different journal, will examine commonalities between PFS and post-SSRI sexual dysfunction (PSSD). That one will be submitted for review before October 1.
The third study, which we announced in October, identified via a next-generation sequencing technology known as RNA-seq, several genes that are likely responsible for the side effects observed in PFS patients. Melcangi notes that though the analysis of this research was particularly complex, he finally got a handle on it this summer, and he’s aiming to submit it to yet another journal by November 1.
As usual, please be aware that there’s never any guarantee that research from any institution will be published in any medical journal.
THE MYTH OF LIQUID FIN
Just when we thought the world was coming around to the fact that finasteride can and does in a subset of men lead to a laundry list of persistent side effects that reads like something Dr. Josef Mengele cooked up, along comes another Big Lie.
 That would be topical finasteride.
That would be topical finasteride.
In February, Deutsche Apotheker Zeitung (DAZ), Germany’s oldest and most widely read pharmaceutical journal, ran a story headlined Is Topical Finasteride a Better Alternative to Treat Alopecia? (English). The article, by DAZ Drugs & Therapy Editor Desiree Aberle—a pharmacist herself—was prompted by the launch of Germany’s first topical-finasteride product, Fynzur for Men, a prescription-only spray from Swiss pharmaceutical company Laboratoires Bailleul. Aberle wrote:
In some cases, [side effects], including insomnia or anhedonia, can persist even years after discontinuing finasteride, known as post-finasteride syndrome. This syndrome is highly distressing for those affected, and there are currently no effective treatment options available. Therefore, it is worth considering whether local application to the scalp could reduce the risk of side effects.
Fynzur, she reported, is being touted as probably safer than oral finasteride, based on a 2021 study titled Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial:
The authors…conclude that the likelihood of systemic side effects is lower with topical therapy. However, the…Post-Finasteride Syndrome Foundation has case reports on PFS with only topical use of finasteride, so patients should still be made aware of this possibility.
Aberle was correct. Since our 2012 founding, we’ve been contacted by about 3,000 desperate PFS patients seeking help. Of those patients, 13 (0.4%) to date have told us they developed the condition from using topical finasteride only.
A month after Aberle’s report, DAZ published a meta-analysis by Tony Daubitz, PhD, titled Side Effects: Hair Growth with Consequences (English abstract).
In all, Daubitz cited from 28 clinical studies, including those by Prof. Melcangi, Mohit Khera, MD, of Baylor College of Medicine, Michael S. Irwig, MD, of Harvard Medical School, and Abdulmaged M. Traish, PhD, of Boston University School of Medicine. After laying out evidence supporting the hypothesis that a proportion of all patients taking oral finasteride develop PFS, Dr. Daubitz wrote:
 A new treatment alternative [to oral finasteride] is topical finasteride (Fynzur, 2275 mg/ml), which was recently launched in Germany. In a phase III study with 250 volunteers [Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia], the spray significantly stimulated hair growth…and showed similar effects to oral finasteride… On the other hand, the systemic drug exposure with dermal application was less than one hundredth of that with oral application. The DHT levels in the blood also fell correspondingly less…which is why fewer side effects are expected.
A new treatment alternative [to oral finasteride] is topical finasteride (Fynzur, 2275 mg/ml), which was recently launched in Germany. In a phase III study with 250 volunteers [Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia], the spray significantly stimulated hair growth…and showed similar effects to oral finasteride… On the other hand, the systemic drug exposure with dermal application was less than one hundredth of that with oral application. The DHT levels in the blood also fell correspondingly less…which is why fewer side effects are expected.
So topical finasteride is safer, right? Hardly, wrote Daubitz:
What sounds promising at first glance appears in a different light through an analysis of the drug. It is not possible to say whether the effect of the topical solution is really equivalent to that of the tablet, since the study was not designed to statistically evaluate this endpoint. And that’s just the beginning of its shortcomings. Only 71% of the subjects completed the study at all, and only 55% of the subjects had evaluable photos to assess the success of the therapy.
In a responder analysis commissioned by the [Federal Institute for Drugs and Medical Devices], the subjects were also asked to rate the improvement in their hair growth themselves. However, there were no significant differences in the subjective assessment (improvement in hair growth in 39.8% of men with topical finasteride, 31.0% with oral finasteride and 32.0% with placebo).
He concluded:
It is still unclear to what extent the lower systemic drug exposure translates into a lower risk of side effects. Although sexual side effects were not reported more frequently than with placebo… the limited number of patients in the study…makes it difficult to draw reliable conclusions. Nevertheless, it is important to anticipate the occurrence of such side effects… Although topical finasteride is not approved as a medicinal product in the USA, it is marketed as an over-the-counter medicinal product through telemedicine portals such as ForHims.com. When asked by the DAZ editors about this, the Post-Finasteride Syndrome Foundation points out that they are aware of numerous cases of the condition resulting from topical finasteride application. Further research on the syndrome is therefore urgently needed.
For our complete coverage of the topical-finasteride issue, please see these reports:
Topical Finasteride Could Precipitate PFS, Top German Rx Journal Warns
Study Used to Push Non-FDA-Approved Topical Finasteride Knocked by Top German Rx Journal
VIRTUAL NIGHTMARE
 Meanwhile, more and more PFS patient are telling us that they developed the condition after being prescribed finasteride by one of the Big 3 telemeds—Hims, Keeps and Ro. In October, Chris from Texas wrote:
Meanwhile, more and more PFS patient are telling us that they developed the condition after being prescribed finasteride by one of the Big 3 telemeds—Hims, Keeps and Ro. In October, Chris from Texas wrote:
I’m at my wits-end. I’ve been rapidly experiencing all the symptoms of PFS and in-fact was prescribed it 6 months ago by Keeps… Now it seems my symptoms are only worsening. I’m terrified how much worse this will affect my relationship with my girlfriend. I’ve seen doctors and even received testosterone replacement therapy! I’m only 26 and my entire life…feels stolen from me, all because I didn’t know this was a side effect.
And just last month, Sean from Massachusetts wrote us to say that his son developed PFS after being prescribed topical finasteride by Hims:
He was on finasteride for less than 3 months when multiple symptoms started overnight… The doctors that he’s been dealing with and the websites they’re referring to are telling him his symptoms should go away after 3 months. My son is going up and down mentally, and some days are clearer than others, but he has not at all returned to his former physical self. I hope more people start talking about this subject as the younger generation is so easily influenced by misinformation on the Internet. Just in the last week, I had conversation and convinced 3 other men in their 20s to get off this drug. One of them was already showing early ED signs but didn’t know what was causing it. Another one got the same Hims product 2 weeks earlier and was already peeing blood.
MEDICAL AWARENESS
 We continue to monitor and publish ADR data for finasteride housed in the World Health Organization’s VigiBase database so that health professionals, the public, and members of the media are aware of global trends. Once again, many of the key indicators have risen year over year, with suicidal ideation leading the pack at 13.2%, followed by completed suicide (6.6%), psychiatric disorders (6.1%), and total finasteride ADRs (4.6%). The absolute number of 862 new ADRs during this period translates into about 2.4 likely cases of PFS reported each day to a DRA.
We continue to monitor and publish ADR data for finasteride housed in the World Health Organization’s VigiBase database so that health professionals, the public, and members of the media are aware of global trends. Once again, many of the key indicators have risen year over year, with suicidal ideation leading the pack at 13.2%, followed by completed suicide (6.6%), psychiatric disorders (6.1%), and total finasteride ADRs (4.6%). The absolute number of 862 new ADRs during this period translates into about 2.4 likely cases of PFS reported each day to a DRA.
Clinicians, pharmacologists and researchers, increasingly via social media, continue to voice their concerns about finasteride, particularly for young men.
Year over year, the number of such professionals aggregated on our Doctors & Researchers Speaking Out page rose 21%, from 104 to 126.
Among the new entrants is G. Pérez López, MD, and endocrinologist in Madrid, who in September wrote:
 In December 2018, I treated…a 28-year-old man who was distressed by a number of symptoms associated with finasteride… He had stopped treatment 10 months earlier, but symptoms that affected his quality of life persisted—decreased libido, depression and disassociation “between his brain and his genitals”… From December 2018 until now, I have treated about 50 young men from all over Spain who have been on finasteride for alopecia and whose symptoms persisted in the physical, sexual and psychological spheres.
In December 2018, I treated…a 28-year-old man who was distressed by a number of symptoms associated with finasteride… He had stopped treatment 10 months earlier, but symptoms that affected his quality of life persisted—decreased libido, depression and disassociation “between his brain and his genitals”… From December 2018 until now, I have treated about 50 young men from all over Spain who have been on finasteride for alopecia and whose symptoms persisted in the physical, sexual and psychological spheres.
Four months later, Ahmed S. Zugail, MD, a urologist in Saudi Arabia, tweeted:
Beware of post-finasteride syndrome, tell your patients about it before prescription. Targeted education of prescribing doctors is the key.
Numerous studies show that more than half of the population that uses [finasteride] has some psychiatric pathology, anxiety and depression, insomnia, suicide risk, and/or sexual dysfunction… Likewise, if the patient has some underlying psychiatric pathology or if he has a family history for the same reason, he is at risk of developing [PFS].
MEDIA AWARENESS
Teutonic TV talks PFS (again)
 In October, Dürfen Die Das? (Can They Do That?), a newsmagazine show on German public broadcaster NDR, debuted Finasteride: Why Is This Hair-loss Drug Still on the Market? (English). It marked the fourth German-language PFS report on network TV in just 18 months. The first thee were: Suddenly Bald, Tricks of the Beauty Industry, and The Side Effects of Finasteride Are Underestimated.
In October, Dürfen Die Das? (Can They Do That?), a newsmagazine show on German public broadcaster NDR, debuted Finasteride: Why Is This Hair-loss Drug Still on the Market? (English). It marked the fourth German-language PFS report on network TV in just 18 months. The first thee were: Suddenly Bald, Tricks of the Beauty Industry, and The Side Effects of Finasteride Are Underestimated.
Hosted by Neils Walker, the 17-minute film featured interviews with two PFS patients, Sebastian and Andreas. “I was on the brink of jumping off a bridge,” said Sebastian. “From the moment the disorder started, I’ve never been able to sleep properly.”
“The worst for me was the emotional stress, which has been so bad that I would have taken my own life if it had continued,” said Andreas. “Suffering from PFS is like all the life and joy has been sucked out of you.”
Also featured was Hartmut Porst, MD, founder of the European Institute for Sexual Health, who told Walker, “In my career, I’ve lost two patients who became suicidal. [Finasteride] should have been pulled from the market at least 10 years ago.”
Parents go public
In Dr. Khera’s groundbreaking PFS research, published in June 2020, he wrote, “Two patients (8%) in the 5ARI arm committed suicide during or after the study period.” At the time, only one of those patients, Daniel Stewart, was known to the public. Because shortly after his death in 2014, parents Glenn and Rita Stewart, asked us to share the heartbreaking news of his passing.
 Eight years later, the parents of the other late patient in Dr. Khera’s study joined the Stewarts’ struggle when the New York Post published a feature headlined, Propecia users claim drug causes memory loss, ED, suicidal thoughts. In it, reporter Michael Kaplan revealed that Stephen Kenney, a decorated Atlanta detective whose work had been featured on the A&E news magazine The First 48, took his own life in 2014. He was 42, and had been suffering from PFS for three years.
Eight years later, the parents of the other late patient in Dr. Khera’s study joined the Stewarts’ struggle when the New York Post published a feature headlined, Propecia users claim drug causes memory loss, ED, suicidal thoughts. In it, reporter Michael Kaplan revealed that Stephen Kenney, a decorated Atlanta detective whose work had been featured on the A&E news magazine The First 48, took his own life in 2014. He was 42, and had been suffering from PFS for three years.
“There was insomnia, fatigue, sexual dysfunction and numbness in the pelvic region,” dad Bob Kenney, told The Post. “Doctors doing the study at Baylor said that his problems were finasteride-related.”
“Steve went from optimistic to morose,” said mom Kathleen Kenney of the impact Propecia had on her son. “How many people like us had to read suicide notes from their sons, saying they are sorry for putting us through this?”
(For a complete listing of PFS press coverage, please visit our PFS Media Awareness page.)
SOCIAL MEDIA & CONTENT MARKETING
Nine months ago, our Facebook page, which we’d launched in 2014, mysteriously disappeared. Or so we thought. What happened was, sometime in 2022, Facebook required all businesses, including nonprofits, to shift to a Facebook for Business (FFB) account, or be deleted. But we never got that memo. So Facebook nixed us. Fortunately, we were able to open a proper FFB account, and repost our old content. The one hitch is that it’s at a different URL. So if you had been following us on our old Facebook page, you’ll need to follow us from scratch on the new one. And please tell everyone you know to do the same.
Now, in the four months it took to rebuild our page, we took the opportunity to craft a new, more urgent tagline: “Know the risks.” That got us thinking we ought to do a better job of promoting key sections of our website. So we developed a series of eight slides that now sit atop our homepage, each linking to important content. For anyone motivated enough to help spread the word, feel free to grab hi-res versions of the slides from the links below, for sharing via your own social pages. Just be sure to link each image back to the respective pages on our site:
 Got PFS?, Take the PFS Challenge, Feds fumble deathly disclosure, Don’t be caught tele-dead, This is your body. This is your body on finasteride, Precisely the same syndrome, Only a madman could have imagined, Furious at finasteride?
Got PFS?, Take the PFS Challenge, Feds fumble deathly disclosure, Don’t be caught tele-dead, This is your body. This is your body on finasteride, Precisely the same syndrome, Only a madman could have imagined, Furious at finasteride?
COLD CASE #1
In our database of about 3,000 PFS patients, amassed over 11 years, are 19 cases of suicide (0.6%). That means for every 1,000 people who take finasteride and develop persistent side effects, six kill themselves. True, that statistic wouldn’t pass peer review for publication in the New England Journal of Medicine. But it can’t be easily dismissed either.
You see, of those 19 suicides, eight were copiously documented for inclusion in our 2017 FDA Citizen Petition, which prompted the agency to add suicidality to the Propecia product label. Another two were fairly well documented, but we opted not to include them in the petition, lest the FDA use some slight weaknesses to toss the entire petition.
That leaves nine cases. Of them, one was reported to us by the estranged wife of the PFS patient, two by the brothers of the patients, two by the mothers of the patients, two by the fathers of the patients, and two were PFS patients who told us directly they felt like killing themselves, then did so shortly after.
 That’s a scary track record for any prescription drug, let alone one most commonly used for cosmetic purposes.
That’s a scary track record for any prescription drug, let alone one most commonly used for cosmetic purposes.
Scarier still is this: I’m nearly certain that there aren’t 19 suicide cases total in our database. I’m nearly certain that there are 21, which would mean for every 1,000 people who take finasteride and develop persistent side effects, seven kill themselves.
I came to this opinion earlier in the year while inputting all 3,000 case reports into a customer relationship management (CRM) database, which allows us to better slice and dice that data. In the case of one patient logged only as “J Dunford” from the UK, I noticed that, from September 2016 to September 2017, he made six monetary donations to the PFSF. Then he stopped cold. He also read the PFSF newsfeed from March 11 through December 20 of 2017. Then he stopped cold.
Just out of curiosity, I Googled “J Dunford obituaries” in the UK and it turns out a James Dunford of Warkworth, England—about 90 miles south of Edinburgh, Scotland—went missing on Dec. 28, 2017. The next day, at age 34, he was found dead. There was “not believed to be any third-party involvement” in his death.
Now bells are going off in my head because the very first news item logged on our Media Awareness page is a 2010 BBC News report about a PFS patient from Edinburgh. He was 26 at that time, which would make him 34 in 2017.
And his name was James. And from behind, as pictured in the BBC report, he looked very much like a picture of James Dunford in the Gateshead News blog that reported him missing.
Thinking that James’ family might have no idea he was ever in touch with the PFSF, but might welcome the closure of knowing that finasteride likely led to his suicide—rather than living the rest of their lives wondering why he did it—I tried tracking them down. But no such luck.
I also reached out to the local reporters who covered his death, thinking they might get word to the family. But again no luck.
So for the moment, J Dunford remains one of two cold cases in our database of 3,000 PFS patients.
At least two.
 As we embark on Year 12 of our mission, we ask you to continue giving generously to the foundation so we may continue this urgent work.
As we embark on Year 12 of our mission, we ask you to continue giving generously to the foundation so we may continue this urgent work.
In the meantime, as directed on our Report Your Side Effects page, anyone living in the US who suffers from PFS should report his or her symptoms to the FDA. Anyone living outside the US who suffers from the condition should also report to the FDA, as well as to his or her local DRA.
Finally, if you or a loved one are suffering from PFS and feeling depressed or unstable, please don’t hesitate to contact us via our Patient Support hotline: social@pfsfoundation.org
Sincerely,
Philip Recchia
President
Related News
2022 PFS Foundation Annual Address
2021 PFS Foundation Annual Address
2020 PFS Foundation Annual Address
2019 PFS Foundation Annual Address
2018 PFS Foundation Annual Address
2017 PFS Foundation Annual Address
2016 PFS Foundation Annual Address
2015 PFS Foundation Annual Address
2014 PFS Foundation Annual Address
2013 PFS Foundation Annual Address
